Evaluating case management for caregivers of children with spinal muscular atrophy type I and II-an exploratory, controlled, mixed-methods trial
- PMID: 37808564
- PMCID: PMC10552854
- DOI: 10.3389/fped.2023.1212012
Evaluating case management for caregivers of children with spinal muscular atrophy type I and II-an exploratory, controlled, mixed-methods trial
Abstract
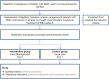
Introduction: Spinal muscular atrophy (SMA) is a rare neuromuscular disease requiring various clinical specialists and therapists to provide care. Due to the disease's dynamic nature and the long distances between specialized centers and local providers, integrating care between disciplines can be challenging. Care that is inadequately integrated can compromise the quality of care and become a burden for patients and families. This trial aimed to improve the care of patients through a case management (CM) intervention.
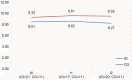
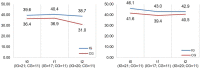
Methods: We conducted an exploratory, controlled, two-arm trial with pre-, post-, and follow-up measures (process and outcome evaluation). Proof of efficacy based on statistical significance was not our primary study objective since we were investigating a rare disease. Primary outcomes were caregivers' HRQoL and caregiver-rated quality of care integration. Our secondary outcome was the children's HRQoL.
Results: Questionnaires and semi-structured interviews yielded heterogeneous results depending on caregivers' level of experience and desire (or possibility) to delegate care tasks.
Discussion: Despite differing perceptions, all participants supported the establishment of a care coordination model. We recommend CM immediately after diagnosis to provide the greatest benefit to families. We hope that our trial will support the further development of CM interventions that can be customized for specific diseases.
Keywords: case management; health-related quality of life; integrated care; rare diseases; spinal muscular atrophy.
© 2023 Willems, Pechmann, Wider, Ambs, Meyer, Cascante, Sproß, Mund, Farin-Glattacker and Langer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Dabbous O, Maru B, Jansen JP, Lorenzi M, Cloutier M, Guérin A, et al. Survival, motor function, and motor milestones: comparison of AVXS-101 relative to nusinersen for the treatment of infants with spinal muscular atrophy type 1. Adv Ther. (2019) 36(5):1164–76. 10.1007/s12325-019-00923-8 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

