This is a preprint.
Cell autonomous polarization by the planar cell polarity signaling pathway
- PMID: 37808631
- PMCID: PMC10557733
- DOI: 10.1101/2023.09.26.559449
Cell autonomous polarization by the planar cell polarity signaling pathway
Abstract
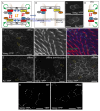
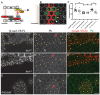
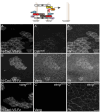
Planar Cell Polarity (PCP) signaling polarizes epithelial cells in a plane orthogonal to their apical-basal axis. A core PCP signaling module segregates two distinct molecular subcomplexes to opposite sides of cells and coordinates the direction of polarization between neighboring cells. Homodimers of the atypical cadherin Flamingo are thought to scaffold these subcomplexes and are required for intercellular polarity signaling. Feedback is required for polarization, but whether feedback requires intercellular and/or intracellular pathways is unknown, and traditional genetic tools have limited utility in dissecting these mechanisms. Using novel tools, we show that cells lacking Flamingo, or bearing a homodimerization-deficient Flamingo, do polarize, indicating that functional PCP subcomplexes form and segregate cell-autonomously. We identify feedback pathways and propose a competitive binding-based asymmetry amplifying mechanism that each operate cell-autonomously. The intrinsic logic of PCP signaling is therefore more similar to that in single cell polarizing systems than was previously recognized.
Conflict of interest statement
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
A cross-species analysis of neuroanatomical covariance sex differences in humans and mice.Biol Sex Differ. 2025 Jul 1;16(1):47. doi: 10.1186/s13293-025-00728-1. Biol Sex Differ. 2025. PMID: 40598550 Free PMC article.
-
Interventions to reduce harm from continued tobacco use.Cochrane Database Syst Rev. 2016 Oct 13;10(10):CD005231. doi: 10.1002/14651858.CD005231.pub3. Cochrane Database Syst Rev. 2016. PMID: 27734465 Free PMC article.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
References
-
- Chae J., Kim M.J., Goo J.H., Collier S., Gubb D., Charlton J., Adler P.N., and Park W.J. (1999). The Drosophila tissue polarity gene starry night encodes a member of the protocadherin family. Development 126, 5421–5429. - PubMed
-
- Usui T., Shima Y., Shimada Y., Hirano S., Burgess R.W., Schwarz T.L., Takeichi M., and Uemura T. (1999). Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell 98, 585–595. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
