This is a preprint.
Replenishing Age-Related Decline of IRAK-M Expression in Retinal Pigment Epithelium Attenuates Outer Retinal Degeneration
- PMID: 37808640
- PMCID: PMC10557650
- DOI: 10.1101/2023.09.27.559733
Replenishing Age-Related Decline of IRAK-M Expression in Retinal Pigment Epithelium Attenuates Outer Retinal Degeneration
Update in
-
Replenishing IRAK-M expression in retinal pigment epithelium attenuates outer retinal degeneration.Sci Transl Med. 2024 Jun 5;16(750):eadi4125. doi: 10.1126/scitranslmed.adi4125. Epub 2024 Jun 5. Sci Transl Med. 2024. PMID: 38838135
Abstract
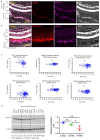
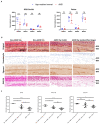
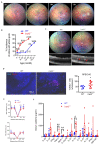
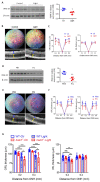
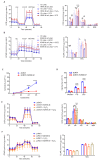
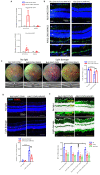
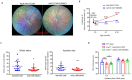
Unchecked, chronic inflammation is a constitutive component of age-related diseases, including age-related macular degeneration (AMD). Here we identified interleukin-1 receptor-associated kinase (IRAK)-M as a key immunoregulator in retinal pigment epithelium (RPE) that declines with age. Rare genetic variants of IRAK-M increased the likelihood of AMD. IRAK-M expression in RPE declined with age or oxidative stress and was further reduced in AMD. IRAK-M-deficient mice exhibited increased incidence of outer retinal degeneration at earlier ages, which was further exacerbated by oxidative stressors. The absence of IRAK-M disrupted RPE cell homeostasis, including compromised mitochondrial function, cellular senescence, and aberrant cytokine production. IRAK-M overexpression protected RPE cells against oxidative or immune stressors. Subretinal delivery of AAV-expressing IRAK-M rescued light-induced outer retinal degeneration in wild-type mice and attenuated age-related spontaneous retinal degeneration in IRAK-M-deficient mice. Our data support that replenishment of IRAK-M expression may redress dysregulated pro-inflammatory processes in AMD, thereby treating degeneration.
Conflict of interest statement
Competing interests ADD, JL and YKC are named inventors on an International Patent Application No: PCT/EP2022/082518. ADD is consultant for Hubble Tx, Affibody, 4 DMT, Novartis, Roche, UCB, Amilera, Janssen, and ActivBio. RG is consultant for Roche, Genentech, Apellis, Novartis, and Bayer.
Figures
References
-
- Franceschi C., Garagnani P., Parini P., Giuliani C., Santoro A., Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 14, 576–590 (2018). - PubMed
-
- Copland D. A., Theodoropoulou S., Liu J., Dick A. D., A Perspective of AMD Through the Eyes of Immunology. Invest Ophthalmol Vis Sci 59, AMD83–AMD92 (2018). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
