This is a preprint.
Integrated single-cell multiome analysis reveals muscle fiber-type gene regulatory circuitry modulated by endurance exercise
- PMID: 37808658
- PMCID: PMC10557702
- DOI: 10.1101/2023.09.26.558914
Integrated single-cell multiome analysis reveals muscle fiber-type gene regulatory circuitry modulated by endurance exercise
Update in
-
Integrated single-cell multiome analysis reveals muscle fiber-type gene regulatory circuitry modulated by endurance exercise.Genome Res. 2025 Jul 1;35(7):1664-1677. doi: 10.1101/gr.280051.124. Genome Res. 2025. PMID: 40393809
Abstract
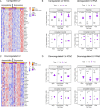
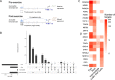
Endurance exercise is an important health modifier. We studied cell-type specific adaptations of human skeletal muscle to acute endurance exercise using single-nucleus (sn) multiome sequencing in human vastus lateralis samples collected before and 3.5 hours after 40 min exercise at 70% VO2max in four subjects, as well as in matched time of day samples from two supine resting circadian controls. High quality same-cell RNA-seq and ATAC-seq data were obtained from 37,154 nuclei comprising 14 cell types. Among muscle fiber types, both shared and fiber-type specific regulatory programs were identified. Single-cell circuit analysis identified distinct adaptations in fast, slow and intermediate fibers as well as LUM-expressing FAP cells, involving a total of 328 transcription factors (TFs) acting at altered accessibility sites regulating 2,025 genes. These data and circuit mapping provide single-cell insight into the processes underlying tissue and metabolic remodeling responses to exercise.
Keywords: PPARδ; acute endurance exercise; circadian control; epigenomics; gene regulatory circuits; human vastus lateralis; single-nucleus multiome; skeletal muscle; snRNA-seq/snATAC-seq; transcriptomics.
Conflict of interest statement
Declaration of Interests: SCS is interim Chief Scientific Officer, consultant, and equity owner of GNOMX Corp. The authors declare no other competing interests.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
