This is a preprint.
Tenofovir Activation is Diminished in the Brain and Liver of Creatine Kinase Brain-Type Knockout Mice
- PMID: 37808667
- PMCID: PMC10557616
- DOI: 10.1101/2023.09.25.559370
Tenofovir Activation is Diminished in the Brain and Liver of Creatine Kinase Brain-Type Knockout Mice
Update in
-
Tenofovir Activation Is Diminished in the Brain and Liver of Creatine Kinase Brain-Type Knockout Mice.ACS Pharmacol Transl Sci. 2024 Jan 3;7(1):222-235. doi: 10.1021/acsptsci.3c00250. eCollection 2024 Jan 12. ACS Pharmacol Transl Sci. 2024. PMID: 38230280 Free PMC article.
Abstract
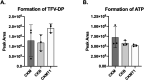
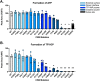
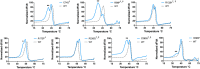
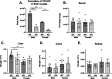
Tenofovir (TFV) is a nucleotide reverse transcriptase inhibitor prescribed for the treatment and prevention of human immunodeficiency virus infection, and the treatment of chronic hepatitis B virus infection. Here, we demonstrate that creatine kinase brain-type (CKB) can form tenofovir-diphosphate (TFV-DP), the pharmacologically active metabolite, in vitro, and identify nine missense mutations (C74S, R96P, S128R, R132H, R172P, R236Q, C283S, R292Q, and H296R) that diminish this activity. Additional characterization of these mutations reveal that five (R96P, R132H, R236Q, C283S, and R292Q) have ATP dephosphorylation catalytic efficiencies less than 20% of wild-type (WT), and seven (C74S, R96P, R132H, R172P, R236Q, C283S, and H296P) induce thermal instabilities. To determine the extent CKB contributes to TFV activation in vivo, we generated a CKB knockout mouse strain, Ckbtm1Nnb. Using an in vitro assay, we show that brain lysates of Ckbtm1Nnb male and female mice form 70.5% and 77.4% less TFV-DP than wild-type brain lysates of the same sex, respectively. Additionally, we observe that Ckbtm1Nnb male mice treated with tenofovir disoproxil fumarate for 14 days exhibit a 22.8% reduction in TFV activation in liver compared to wild-type male mice. Lastly, we utilize mass spectrometry-based proteomics to elucidate the impact of the knockout on the abundance of nucleotide and small molecule kinases in the brain and liver, adding to our understanding of how loss of CKB may be impacting tenofovir activation in these tissues. Together, our data suggest that disruptions in CKB may lower levels of active drug in brain and liver.
Keywords: creatine kinase brain-type; drug metabolism; knockout; mouse model; proteomics; tenofovir.
Figures

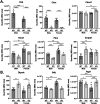
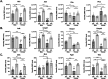
References
-
- Viread (tenofovir disoproxil fumarate) [packet insert]. Foster City, CA: Gilead Sciences, Inc; 2021.
-
- Truvada (emtricitabine/tenofovir disoproxil fumarate) [package insert]; Foster City, CA; Gilead Sciences, Inc; 2022.
-
- Descovy (emtricitabine/tenofovir alafenamide) [package insert]; Foster City, CA; Gilead Sciences, Inc; 2022.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
