This is a preprint.
Sleep and circadian rhythm disruption by NPTX2 loss of function
- PMID: 37808783
- PMCID: PMC10557648
- DOI: 10.1101/2023.09.26.559408
Sleep and circadian rhythm disruption by NPTX2 loss of function
Abstract
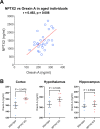
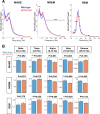
Sleep and circadian rhythm disruption (SCRD) is commonly observed in aging, especially in individuals who experience progressive cognitive decline to mild cognitive impairment (MCI) and Alzheimer's disease (AD). However, precise molecular mechanisms underlying the association between SCRD and aging are not fully understood. Orexin A is a well-characterized "sleep neuropeptide" that is expressed in hypothalamic neurons and evokes wake behavior. The importance of Orexin is exemplified in narcolepsy where it is profoundly down-regulated. Interestingly, the synaptic immediate early gene NPTX2 is co-expressed in Orexin neurons and is similarly reduced in narcolepsy. NPTX2 is also down-regulated in CSF of some cognitively normal older individuals and predicts the time of transition from normal cognition to MCI. The association between Orexin and NPTX2 is further evinced here where we observe that Orexin A and NPTX2 are highly correlated in CSF of cognitively normal aged individuals and raises the question of whether SCRD that are typically attributed to Orexin A loss of function may be modified by concomitant NPTX2 down-regulation. Is NPTX2 an effector of sleep or simply a reporter of orexin-dependent SCRD? To address this question, we examined NPTX2 KO mice and found they retain Orexin expression in the brain and so provide an opportunity to examine the specific contribution of NPTX2 to SCRD. Our results reveal that NPTX2 KO mice exhibit a disrupted circadian onset time, coupled with increased activity during the sleep phase, suggesting difficulties in maintaining states. Sleep EEG indicates distinct temporal allocation shifts across vigilance states, characterized by reduced wake and increased NREM time. Evident sleep fragmentation manifests through alterations of event occurrences during Wake and NREM, notably during light transition periods, in conjunction with an increased frequency of sleep transitions in NPTX2 KO mice, particularly between Wake and NREM. EEG spectral analysis indicated significant shifts in power across various frequency bands in the wake, NREM, and REM states, suggestive of disrupted neuronal synchronicity. An intriguing observation is the diminished occurrence of sleep spindles, one of the earliest measures of human sleep disruption, in NPTX2 KO mice. These findings highlight the effector role of NPTX2 loss of function as an instigator of SCRD and a potential mediator of sleep disruption in aging.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures




References
-
- Allocca G., Ma S., Martelli D., Cerri M., Del Vecchio F., Bastianini S., Zoccoli G., Amici R., Morairty S. R., Aulsebrook A. E., Blackburn S., Lesku J. A., Rattenborg N. C., Vyssotski A. L., Wams E., Porcheret K., Wulff K., Foster R., Chan J. K. M., Nicholas C. L., Freestone D. R., Johnston L. A., and Gundlach A. L.. 2019. ‘Validation of ‘Somnivore’, a Machine Learning Algorithm for Automated Scoring and Analysis of Polysomnography Data’, Front Neurosci, 13: 207. - PMC - PubMed
-
- Aryal S., Bonanno K., Song B., Mani D. R., Keshishian H., Carr S. A., Sheng M., and Dejanovic B.. 2023. ‘Deep proteomics identifies shared molecular pathway alterations in synapses of patients with schizophrenia and bipolar disorder and mouse model’, Cell Rep, 42: 112497. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
