This is a preprint.
Identification of host factors for Rift Valley Fever Phlebovirus
- PMID: 37808812
- PMCID: PMC10557628
- DOI: 10.1101/2023.09.28.559935
Identification of host factors for Rift Valley Fever Phlebovirus
Update in
-
Identification of Host Factors for Rift Valley Fever Phlebovirus.Viruses. 2023 Nov 13;15(11):2251. doi: 10.3390/v15112251. Viruses. 2023. PMID: 38005928 Free PMC article.
Abstract
Background: Rift Valley fever phlebovirus (RVFV) is a zoonotic pathogen that causes Rift Valley fever (RVF) in livestock and humans. Currently, there is no licensed human vaccine or antiviral drug to control RVF. Although multiple species of animals and humans are vulnerable to RVFV infection, host factors affecting susceptibility are not well understood.
Methodology: To identify the host factors or genes essential for RVFV replication, we conducted a CRISPR-Cas9 knock-out screen in human A549 cells. We then validated the putative genes using siRNA-mediated knockdowns and CRISPR-Cas9-mediated knockout studies, respectively. The role of a candidate gene in the virus replication cycle was assessed by measuring intracellular viral RNA accumulation, and the virus titers by plaque assay or TCID50 assay.
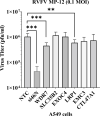
Findings: We identified approximately 900 genes with potential involvement in RVFV infection and replication. Further evaluation of the effect of six genes on viral replication using siRNA-mediated knockdowns found that silencing two genes (WDR7 and LRP1) significantly impaired RVFV replication. For further analysis, we focused on the WDR7 gene since the role of LRP1 in RVFV replication was previously described in detail. Knock-out A549 cell lines were generated and used to dissect the effect of WRD7 on RVFV and another bunyavirus, La Crosse encephalitis virus (LACV). We observed significant effects of WDR7 knock-out cells on both intracellular RVFV RNA levels and viral titers. At the intracellular RNA level, WRD7 affected RVFV replication at a later phase of its replication cycle (24h) when compared to LACV which was affected an earlier replication phase (12h).
Conclusion: In summary, we have identified WDR7 as an essential host factor for the replication of two relevant bunyaviruses, RVFV and LACV. Future studies will investigate the mechanistic role by which WDR7 facilitates Phlebovirus replication.
Keywords: A549 cells; LACV; MP-12; RVFV; WDR7; bunyavirus; host factor; phlebovirus.
Conflict of interest statement
Conflict of Interest All authors declare no conflict of interest. The JAR laboratory received support from Tonix Pharmaceuticals, Xing Technologies, Esperobvax, and Zoetis, outside of the reported work. JAR is inventor of patents and patent applications on the use of antivirals and vaccines for the treatment and prevention of virus infections, owned by Kansas State University, KS.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
