This is a preprint.
Modeling the Transmission Mitigation Impact of Testing for Infectious Diseases
- PMID: 37808825
- PMCID: PMC10557819
- DOI: 10.1101/2023.09.22.23295983
Modeling the Transmission Mitigation Impact of Testing for Infectious Diseases
Update in
-
Modeling the transmission mitigation impact of testing for infectious diseases.Sci Adv. 2024 Jun 14;10(24):eadk5108. doi: 10.1126/sciadv.adk5108. Epub 2024 Jun 14. Sci Adv. 2024. PMID: 38875334 Free PMC article.
Abstract
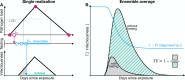
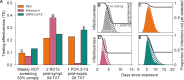
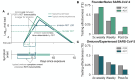
A fundamental question of any program focused on the testing and timely diagnosis of a communicable disease is its effectiveness in reducing transmission. Here, we introduce testing effectiveness (TE)-the fraction by which testing and post-diagnosis isolation reduce transmission at the population scale-and a model that incorporates test specifications and usage, within-host pathogen dynamics, and human behaviors to estimate TE. Using TE to guide recommendations, we show that today's rapid diagnostics should be used immediately upon symptom onset to control influenza A and respiratory syncytial virus (RSV), but delayed by up to 2d to control omicron-era SARS-CoV-2. Furthermore, while rapid tests are superior to RT-qPCR for control of founder-strain SARS-CoV-2, omicron-era changes in viral kinetics and rapid test sensitivity cause a reversal, with higher TE for RT-qPCR despite longer turnaround times. Finally, we illustrate the model's flexibility by quantifying tradeoffs in the use of post-diagnosis testing to shorten isolation times.
Conflict of interest statement
Competing Interests D.B.L. is a member of the scientific advisory board of Darwin BioSciences and discloses past consulting for Flambeau RapidX. The authors declare no other competing interests.
Figures


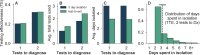
Similar articles
-
Modeling the transmission mitigation impact of testing for infectious diseases.Sci Adv. 2024 Jun 14;10(24):eadk5108. doi: 10.1126/sciadv.adk5108. Epub 2024 Jun 14. Sci Adv. 2024. PMID: 38875334 Free PMC article.
-
Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
References
-
- Pavelka Martin, Kevin Van-Zandvoort Sam Abbott, Sherratt Katharine, Majdan Marek, CMMID COVID-19 working group, Inštitút Zdravotných Analýz, Pavol Jarčuška, Marek Krajčí, Stefan Flasche, and Sebastian Funk. The impact of population-wide rapid antigen testing on SARS-CoV-2 prevalence in Slovakia. Science, 372(6542):635–641, 2021. - PMC - PubMed
-
- Bernstein David I, Mejias Asuncion, Rath Barbara, Woods Christopher W, and Deeter Jamie Phillips. Summarizing study characteristics and diagnostic performance of commercially available tests for respiratory syncytial virus: A scoping literature review in the COVID-19 era. The Journal of Applied Laboratory Medicine, 8(2):353–371, 2023. - PMC - PubMed
-
- Merckx Joanna, Wali Rehab, Schiller Ian, Caya Chelsea, Gore Genevieve C, Chartrand Caroline, Dendukuri Nandini, and Papenburg Jesse. Diagnostic accuracy of novel and traditional rapid tests for influenza infection compared with reverse transcriptase polymerase chain reaction: a systematic review and meta-analysis. Annals of Internal Medicine, 167(6):394–409, 2017. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
