Alterations of oral microbiota are associated with the development and severity of acute pancreatitis
- PMID: 37808891
- PMCID: PMC10557549
- DOI: 10.1080/20002297.2023.2264619
Alterations of oral microbiota are associated with the development and severity of acute pancreatitis
Abstract
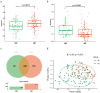
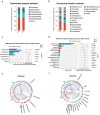
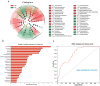
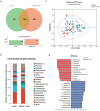
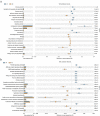
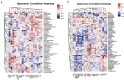
Acute pancreatitis (AP) is a common abdomen clinical emergency. Most APs have mild clinical symptoms and a good prognosis. However, about 20% of patients develop severe acute pancreatitis (SAP), increasing morbidity and mortality. The microbiome's impact on AP pathophysiology has received increasing attention. Hence, to explore changes in oral microbial composition in acute pancreatitis, we collected clinical information and oral saliva samples from 136 adult participants: 47 healthy controls, 43 acute mild AP (MAP), 29 moderate AP (MSAP), and 17 severe AP (SAP). Using 16S rRNA gene sequencing, 663,175 high-quality sequences were identified. The relative abundance and diversity of oral microorganisms in AP patients increased, with decreased beneficial bacteria such as Streptococcus, Neisseria, and Gemella, and increased Prevotella, Veillonella, Granulicatella, Actinomyces, and Peptostreptococcus in the AP group. Further changes in microbial composition occurred with increasing disease severity, including a decreased abundance of beneficial bacteria such as Neisseria, Haemophilus, and Gemella in MSAP and SAP compared to MAP. Moreover, the Lefse analysis showed that Prevotella, Peptostreptococcus, Actinomyces, and Porphyromonas were better microbial markers for AP. Therefore, oral microbiome changes could distinguish AP from healthy individuals and serve as an early novel predictor of disease severity in AP patients.
Keywords: 16S rRNA gene; Acute pancreatitis; diversity; dysbiosis; oral microbiota; prediction.
© 2023 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- Vege SS, Gardner TB, Chari ST, et al. Low mortality and high morbidity in severe acute pancreatitis without organ failure: a case for revising the Atlanta classification to include “moderately severe acute pancreatitis”. Am J Gastroenterol. 2009;104(3):710–715. doi: 10.1038/ajg.2008.77 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
