Mucormycosis in 2023: an update on pathogenesis and management
- PMID: 37808914
- PMCID: PMC10552646
- DOI: 10.3389/fcimb.2023.1254919
Mucormycosis in 2023: an update on pathogenesis and management
Abstract
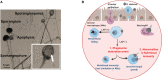
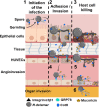
Mucormycosis (MCR) is an emerging and frequently lethal fungal infection caused by the Mucorales family, with Rhizopus, Mucor, and Lichtheimia, accounting for > 90% of all cases. MCR is seen in patients with severe immunosuppression such as those with hematologic malignancy or transplantation, Diabetes Mellitus (DM) and diabetic ketoacidosis (DKA) and immunocompetent patients with severe wounds. The recent SARS COV2 epidemy in India has resulted in a tremendous increase in MCR cases, typically seen in the setting of uncontrolled DM and corticosteroid use. In addition to the diversity of affected hosts, MCR has pleiotropic clinical presentations, with rhino-orbital/rhino-cerebral, sino-pulmonary and necrotizing cutaneous forms being the predominant manifestations. Major insights in MCR pathogenesis have brought into focus the host receptors (GRP78) and signaling pathways (EGFR activation cascade) as well as the adhesins used by Mucorales for invasion. Furthermore, studies have expanded on the importance of iron availability and the complex regulation of iron homeostasis, as well as the pivotal role of mycotoxins as key factors for tissue invasion. The molecular toolbox to study Mucorales pathogenesis remains underdeveloped, but promise is brought by RNAi and CRISPR/Cas9 approaches. Important recent advancements have been made in early, culture-independent molecular diagnosis of MCR. However, development of new potent antifungals against Mucorales remains an unmet need. Therapy of MCR is multidisciplinary and requires a high index of suspicion for initiation of early Mucorales-active antifungals. Reversal of underlying immunosuppression, if feasible, rapid DKA correction and in selected patients, surgical debulking are crucial for improved outcomes.
Keywords: COVID-19-associated mucormycosis; DKA; Mucorales; immunosuppression; invasive fungal infections; mucormycosis; pathogenicity; rhizopus.
Copyright © 2023 Alqarihi, Kontoyiannis and Ibrahim.
Conflict of interest statement
AI has received research support from and served on advisory boards for Amplyx, Astellas, Cidara and Navigen. AI owns shares in Vitalex Biosciences, a start-up company that is developing immunotherapies and diagnostics for MCR. DK reports honoraria and research support from Gilead Sciences and Astellas, Inc, received consultant fees from Astellas Pharma, Merck, and Gilead Sciences, and is a member of the Data Review Committee of Cidara Therapeutics, AbbVie, and the Mycoses Study Group. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures


References
-
- Abzug M. J., Walsh T. J. (2004). Interferon-gamma and colony-stimulating factors as adjuvant therapy for refractory fungal infections in children. Pediatr. Infect. Dis. J. 23 (8), 769–773. doi: 10.1097/01.inf.0000134314.65398.bf - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

