A quantitative systems pharmacology model for certolizumab pegol treatment in moderate-to-severe psoriasis
- PMID: 37809085
- PMCID: PMC10552644
- DOI: 10.3389/fimmu.2023.1212981
A quantitative systems pharmacology model for certolizumab pegol treatment in moderate-to-severe psoriasis
Abstract
Background: Psoriasis is a chronic immune-mediated inflammatory systemic disease with skin manifestations characterized by erythematous, scaly, itchy and/or painful plaques resulting from hyperproliferation of keratinocytes. Certolizumab pegol [CZP], a PEGylated antigen binding fragment of a humanized monoclonal antibody against TNF-alpha, is approved for the treatment of moderate-to-severe plaque psoriasis. Patients with psoriasis present clinical and molecular variability, affecting response to treatment. Herein, we utilized an in silico approach to model the effects of CZP in a virtual population (vPop) with moderate-to-severe psoriasis. Our proof-of-concept study aims to assess the performance of our model in generating a vPop and defining CZP response variability based on patient profiles.
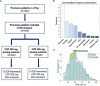
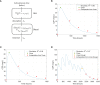
Methods: We built a quantitative systems pharmacology (QSP) model of a clinical trial-like vPop with moderate-to-severe psoriasis treated with two dosing schemes of CZP (200 mg and 400 mg, both every two weeks for 16 weeks, starting with a loading dose of CZP 400 mg at weeks 0, 2, and 4). We applied different modelling approaches: (i) an algorithm to generate vPop according to reference population values and comorbidity frequencies in real-world populations; (ii) physiologically based pharmacokinetic (PBPK) models of CZP dosing schemes in each virtual patient; and (iii) systems biology-based models of the mechanism of action (MoA) of the drug.
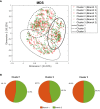
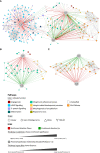
Results: The combination of our different modelling approaches yielded a vPop distribution and a PBPK model that aligned with existing literature. Our systems biology and QSP models reproduced known biological and clinical activity, presenting outcomes correlating with clinical efficacy measures. We identified distinct clusters of virtual patients based on their psoriasis-related protein predicted activity when treated with CZP, which could help unravel differences in drug efficacy in diverse subpopulations. Moreover, our models revealed clusters of MoA solutions irrespective of the dosing regimen employed.
Conclusion: Our study provided patient specific QSP models that reproduced clinical and molecular efficacy features, supporting the use of computational methods as modelling strategy to explore drug response variability. This might shed light on the differences in drug efficacy in diverse subpopulations, especially useful in complex diseases such as psoriasis, through the generation of mechanistically based hypotheses.
Keywords: anti-TNF; certolizumab pegol; mathematical modelling; mechanism of action; psoriasis; virtual population.
Copyright © 2023 Coto-Segura, Segú-Vergés, Martorell, Moreno-Ramírez, Jorba, Junet, Guerri, Daura, Oliva, Cara, Suárez-Magdalena, Abraham and Mas.
Conflict of interest statement
Authors CS-V, GJ, VJ, FG and JM are full-time employees at Anaxomics Biotech. Authors CC and OS-M are full-time employees at UCB Pharma. Author SA was a former employee at UCB Pharma. The authors declare that this study received funding from Anaxomics Biotech. The funder had the following involvement in the study: data analysis.
Figures

References
-
- Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol (2008) 58(5):826–50. - PubMed
-
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med (2009) 361(5):496–509. - PubMed
-
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol (2014) 70(3):512–6. - PubMed
-
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol (2017) 31(2):205–12. - PubMed
-
- Global report on psoriasis. World Health Organization. (2016). Available at: https://apps.who.int/iris/handle/10665/204417.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

