Nb-doped NiO nanoflowers for nitrite electroreduction to ammonia
- PMID: 37810221
- PMCID: PMC10558769
- DOI: 10.1016/j.isci.2023.107944
Nb-doped NiO nanoflowers for nitrite electroreduction to ammonia
Abstract
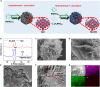
Electrocatalytic reduction of nitrite to ammonia (NO2RR) is considered as an appealing route to simultaneously achieve sustainable ammonia production and abate hazardous nitrite pollution. Herein, atomically Nb-doped NiO nanoflowers are designed as a high-performance NO2RR catalyst, which exhibits the highest NH3-Faradaic efficiency of 92.4% with an NH3 yield rate of 200.5 μmol h-1 cm-2 at -0.6 V RHE. Theoretical calculations unravel that Nb dopants can act as Lewis acid sites to render effective NO2- activation, decreased protonation energy barriers, and restricted hydrogen evolution, ultimately leading to a high NO2RR selectivity and activity.
Keywords: Applied chemistry; Electrochemistry; Energy materials.
© 2023 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Liang J., Li Z., Zhang L., He X., Luo Y., Zheng D., Wang Y., Li T., Yan H., Ying B., et al. Advances in ammonia electrosynthesis from ambient nitrate/nitrite reduction. Chem. 2023;9:1768–1827.
-
- Liang J., Liu Q., Alshehri A.A., Sun X. Recent advances in nanostructured heterogeneous catalysts for N-cycle electrocatalysis. Nano Res. Energy. 2022;1
-
- Qi D., Lv F., Wei T., Jin M., Meng G., Zhang S., Liu Q., Liu W., Ma D., Hamdy M.S., et al. High-efficiency electrocatalytic NO reduction to NH3 by nanoporous VN. Nano Res. Energy. 2022;1
-
- Liu Q., Xu T., Luo Y., Kong Q., Li T., Lu S., Alshehri A.A., Alzahrani K.A., Sun X. Recent advances in strategies for highly selective electrocatalytic N2 reduction toward ambient NH3 synthesis. Curr. Opin. Electrochem. 2021;29
-
- Luo Y., Shen P., Li X., Guo Y., Chu K. Sulfur-deficient Bi2S3−x synergistically coupling Ti3C2Tx-MXene for boosting electrocatalytic N2 reduction. Nano Res. 2022;15:3991–3999.
LinkOut - more resources
Full Text Sources

