The senescent secretome drives PLVAP expression in cultured human hepatic endothelial cells to promote monocyte transmigration
- PMID: 37810232
- PMCID: PMC10558774
- DOI: 10.1016/j.isci.2023.107966
The senescent secretome drives PLVAP expression in cultured human hepatic endothelial cells to promote monocyte transmigration
Abstract
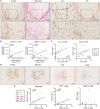
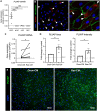
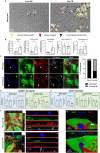
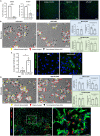
Liver sinusoidal endothelial cells (LSEC) undergo significant phenotypic change in chronic liver disease (CLD), and yet the factors that drive this process and the impact on their function as a vascular barrier and gatekeeper for immune cell recruitment are poorly understood. Plasmalemma-vesicle-associated protein (PLVAP) has been characterized as a marker of LSEC in CLD; notably we found that PLVAP upregulation strongly correlated with markers of tissue senescence. Furthermore, exposure of human LSEC to the senescence-associated secretory phenotype (SASP) led to a significant upregulation of PLVAP. Flow-based assays demonstrated that SASP-driven leukocyte recruitment was characterized by paracellular transmigration of monocytes while the majority of lymphocytes migrated transcellularly. Knockdown studies confirmed that PLVAP selectively supported monocyte transmigration mediated through PLVAP's impact on LSEC permeability by regulating phospho-VE-cadherin expression and endothelial gap formation. PLVAP may therefore represent an endothelial target that selectively shapes the senescence-mediated immune microenvironment in liver disease.
Keywords: Cell biology; Microenvironment; Molecular biology; Omics; Transcriptomics.
© 2023 The Author(s).
Conflict of interest statement
S.S. is a consultant for Faron Pharmaceuticals.
Figures


References
-
- Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S. Burden of liver diseases in the world. J. Hepatol. 2019;70:151–171. - PubMed
-
- Geneva World Health Organisation 2020. https://www.who.int/data/gho/data/themes/mortality-and-global-health-est...
-
- International Agency for Research on Cancer GLOBOCAN . 2018. https://gco.iarc.fr/today/online-analysis-map?v=2020&mode=population&mod...
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

