Pd-Catalyzed Regioselective Cyclopropanation of 2-Substituted 1,3-Dienes
- PMID: 37810406
- PMCID: PMC10557126
- DOI: 10.1021/acsorginorgau.3c00024
Pd-Catalyzed Regioselective Cyclopropanation of 2-Substituted 1,3-Dienes
Abstract
A Pd-catalyzed 3,4-regioselective cyclopropanation of 2-substituted 1,3-dienes by decomposition of diazo esters is reported. The vinylcyclopropanes generated are isolated in practical chemical yields with high levels of regioselectivity but low diastereoselectivity. The system operates under mild reaction conditions, is scalable, and tolerates various sensitive functional groups. A series of original postcatalytic derivatizations is presented to highlight the synthetic potential of the catalytic method.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

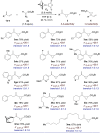
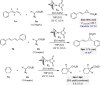


References
-
- Aratani T.; Yoneyoshi Y.; Nagase T. Asymmetric synthesis of chrysanthemic acid. An application of copper carbenoid reaction. Tetrahedron Lett. 1975, 16, 1707–1710. 10.1016/S0040-4039(00)72239-8. - DOI
-
- Aratani T. Catalytic asymmetric synthesis of cyclopropanecarboxylic acids: an application of chiral copper carbenoid reaction. Pure Appl. Chem. 1985, 57, 1839–1844. 10.1351/pac198557121839. - DOI
-
- Doyle M. P.; Protopopova M. N. New Aspects of Catalytic Asymmetric Cyclopropanation. Tetrahedron 1998, 54, 7919–7946. 10.1016/S0040-4020(98)00222-1. - DOI
LinkOut - more resources
Full Text Sources
