Hierarchical self-assembly of a reflectin-derived peptide
- PMID: 37810582
- PMCID: PMC10552760
- DOI: 10.3389/fchem.2023.1267563
Hierarchical self-assembly of a reflectin-derived peptide
Abstract
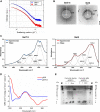
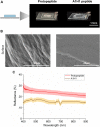
Reflectins are a family of intrinsically disordered proteins involved in cephalopod camouflage, making them an interesting source for bioinspired optical materials. Understanding reflectin assembly into higher-order structures by standard biophysical methods enables the rational design of new materials, but it is difficult due to their low solubility. To address this challenge, we aim to understand the molecular self-assembly mechanism of reflectin's basic unit-the protopeptide sequence YMDMSGYQ-as a means to understand reflectin's assembly phenomena. Protopeptide self-assembly was triggered by different environmental cues, yielding supramolecular hydrogels, and characterized by experimental and theoretical methods. Protopeptide films were also prepared to assess optical properties. Our results support the hypothesis for the protopeptide aggregation model at an atomistic level, led by hydrophilic and hydrophobic interactions mediated by tyrosine residues. Protopeptide-derived films were optically active, presenting diffuse reflectance in the visible region of the light spectrum. Hence, these results contribute to a better understanding of the protopeptide structural assembly, crucial for the design of peptide- and reflectin-based functional materials.
Keywords: bio-based materials; films; hydrogels; optical materials; peptides; reflectins; self-assembly; supramolecular.
Copyright © 2023 Dias, Moreira, Lychko, Lopes Soares, Nurrito, Moura Barbosa, Lutz-Bueno, Mezzenga, Carvalho, Pina and Roque.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
-
- Bastos P., Fracalossi D. M., Chimal M. E., Sánchez A., Rosas C. (2020). Digestive enzymes and timing of digestion in Octopus vulgaris type II. Aquac. Rep. 16, 100262. 10.1016/j.aqrep.2019.100262 - DOI
-
- Berendsen H. J. C., Postma J. P. M., Gunsteren W. F. V., DiNola A., Haak J. R. (1984). Molecular Dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690. 10.1063/1.448118 - DOI
-
- Chen T., Li M., Liu J. (2018). π-π stacking interaction: A nondestructive and facile means in material engineering for bioapplications. Cryst. Growth Des. 18, 2765–2783. 10.1021/acs.cgd.7b01503 - DOI
-
- Chen Y. L., Chang Y. H., Huang J. L., Chen I., Kuo C. (2012). Light scattering and enhanced photoactivities of electrospun titania nanofibers. J. Phys. Chem. C 116, 3857–3865. 10.1021/jp2117246 - DOI
LinkOut - more resources
Full Text Sources

