Synthesis and Studies of Pyridoneimine-Functionalized PETIM Dendrimers
- PMID: 37810657
- PMCID: PMC10552491
- DOI: 10.1021/acsomega.3c03720
Synthesis and Studies of Pyridoneimine-Functionalized PETIM Dendrimers
Abstract
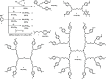
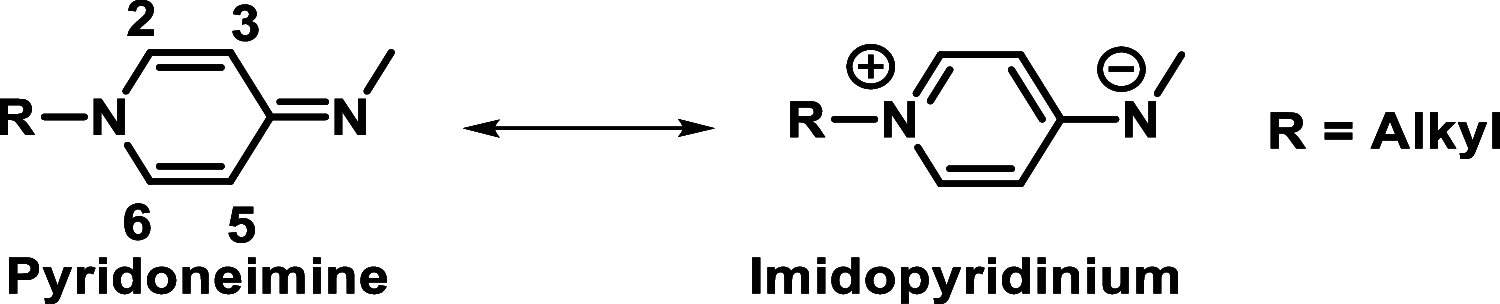
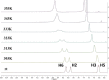
Pyridinoimine-functionalized poly(ether imine) (PETIM) dendrimers of 1-3 generations, possessing 4-16 moieties at the peripheries, are synthesized. Chloride-functionalized dendrimers are reacted with N-methylamino pyridine, under basic conditions, which led to functionalization of the peripheries of a dendrimer with pyridoneimine moieties. Variable-temperature 1H NMR studies are performed to assess the contributing resonance forms of pyridoneimine in the dendrimers. Solvatochromism and 15N NMR studies aid further the assessment of the contributing resonance forms. Comparison with derivatives that possess 1 and 2 pyridoneimines illustrates the contributing resonance forms between nonaromatic pyridoneimine and zwitter ionic aromatic imidopyridinium species.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Malkoch M.; Malmström E.; Nyström A. M.. Dendrimers. In Polymer Science: A Comprehensive Reference; Moeller M.; Matyjaszewski K.. Eds. Elsevier, 2012, Vol. 6, pp 113–176.
LinkOut - more resources
Full Text Sources

