An Update on Strategies to Deliver Protein and Peptide Drugs to the Eye
- PMID: 37810716
- PMCID: PMC10552503
- DOI: 10.1021/acsomega.3c02897
An Update on Strategies to Deliver Protein and Peptide Drugs to the Eye
Abstract
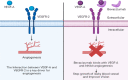
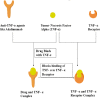
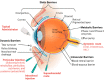
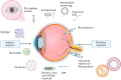
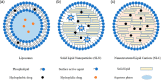
In the past few decades, advancements in protein engineering, biotechnology, and structural biochemistry have resulted in the discovery of various techniques that enhanced the production yield of proteins, targetability, circulating half-life, product purity, and functionality of proteins and peptides. As a result, the utilization of proteins and peptides has increased in the treatment of many conditions, including ocular diseases. Ocular delivery of large molecules poses several challenges due to their high molecular weight, hydrophilicity, unstable nature, and poor permeation through cellular and enzymatic barriers. The use of novel strategies for delivering protein and peptides such as glycoengineering, PEGylation, Fc-fusion, chitosan nanoparticles, and liposomes have improved the efficacy, safety, and stability, which consequently expanded the therapeutic potential of proteins. This review article highlights various proteins and peptides that are useful in ocular disorders, challenges in their delivery to the eye, and strategies to enhance ocular bioavailability using novel delivery approaches. In addition, a few futuristic approaches that will assist in the ocular delivery of proteins and peptides were also discussed.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
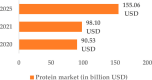
- Therapeutic Proteins Global Market Report 2021: COVID-19 Impact and Recovery to 2030 n.d. https://www.researchandmarkets.com/reports/5319142/therapeutic-proteins-... (accessed April 30, 2022).
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

