Impact of alloying iron pyrite by ruthenium on its band gap values and its insight to photovoltaic performance
- PMID: 37810828
- PMCID: PMC10556601
- DOI: 10.1016/j.heliyon.2023.e20270
Impact of alloying iron pyrite by ruthenium on its band gap values and its insight to photovoltaic performance
Abstract
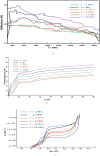
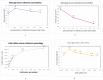
In pursuit of augmenting the band gap value of thin films composed of Pyrite, our study encompasses both theoretical and experimental investigations. Specifically, we sought to delve into the electronic and optical properties of alloyed with ruthenium, denoted as , where x varied across a range of values (x = 0.3966, 0.1586, 0.0496, 0.0347, 0.0106, and 0.00). Our theoretical analysis employed the Linear Muffin-Tin Orbital technique within the Atomic-Sphere approximation (LMTO-ASA) framework, focusing on the density of states. In parallel, our experimental samples were fabricated via a cost-effective and straightforward method involving the sulfuration of amorphous iron oxide thin films, which were deposited through spray pyrolysis of an aqueous solution containing FeCl3.6H2O onto heated glass substrates at 400 °C. This comprehensive investigation sheds light on the influence of alloying on the atomic structure and the optical characteristics of samples. Utilizing X-ray diffraction (XRD) and optical characterizations, we observed a notable widening of the band gap of , ranging from 0.90508 to 1.38 eV, when approximately 1.06% of the Fe atoms were replaced with ruthenium atoms (x = 0.0106 concentration of Ru). This finding holds significant implications for the potential applications of our samples in photovoltaic technologies.
Keywords: Bandgap; Iron pyrite; Optical properties; Photovoltaic cells; Spray pyrolysis.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- itelis A., Vasilakos N., Chalvatzis K. Fostering innovation in renewable energy technologies: choice of policy instruments and effectiveness. Renew. Energy. 2020;151:1163–1172.
-
- Østergaard P.A., Duic N., Noorollahi Y., Mikulcic H., Kalogirou S. Sustainable development using renewable energy technology. Renew. Energy. 2020;146:2430.
-
- Rabaia M.K.H., Abdelkareem M.A., Sayed E.T., Elsaid K., Chae K.-J., Wilberforce T., Olabi A. Environmental impacts of solar energy systems: a review. Sci. Total Environ. 2021;754 - PubMed
-
- Molaei M.J. The optical properties and solar energy conversion applications of carbon quantum dots: a review. Sol. Energy. 2020;196:549–566.
-
- Khalid S., Ahmed E., Khan Y., Riaz K.N., Malik M.A. Nanocrystalline pyrite for photovoltaic applications. ChemistrySelect. 2018;3(23):6488–6524.
LinkOut - more resources
Full Text Sources
Miscellaneous

