Molecular triaging options for women testing HPV positive with self-collected samples
- PMID: 37810963
- PMCID: PMC10560038
- DOI: 10.3389/fonc.2023.1243888
Molecular triaging options for women testing HPV positive with self-collected samples
Abstract
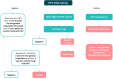
We review developments in molecular triaging options for women who test positive for high-risk human papillomavirus (hrHPV) on self-collected samples in the context of cervical cancer elimination. The World Health Organization (WHO) recommends hrHPV screening as the primary test for cervical screening due to its high sensitivity compared to other screening tests. However, when hrHPV testing is used alone for treatment decisions, a proportion of women of childbearing age receive unnecessary treatments. This provides the incentive to optimize screening regimes to minimize the risk of overtreatment in women of reproductive age. Molecular biomarkers can potentially enhance the accuracy and efficiency of screening and triage. HrHPV testing is currently the only screening test that allows triage with molecular methods using the same sample. Additionally, offering self-collected hrHPV tests to women has been reported to increase screening coverage. This creates an opportunity to focus health resources on linking screen-positive women to diagnosis and treatment. Adding an additional test to the screening algorithm (a triage test) may improve the test's positive predictive value (PPV) and offer a better balance of benefits and risks for women. Conventional triage methods like cytology and visual inspection with acetic acid (VIA) cannot be performed on self-collected samples and require additional clinic visits and subjective interpretations. Molecular triaging using methods like partial and extended genotyping, methylation tests, detection of E6/E7 proteins, and hrHPV viral load in the same sample as the hrHPV test may improve the prediction of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) and invasive cancer, offering more precise, efficient, and cost-effective screening regimes. More research is needed to determine if self-collected samples are effective and cost-efficient for diverse populations and in comparison to other triage methods. The implementation of molecular triaging could improve screening accuracy and reduce the need for multiple clinical visits. These important factors play a crucial role in achieving the global goal of eliminating cervical cancer as a public health problem.
Keywords: Cervical screening; cervical cancer elimination; molecular tests; self-collection; triage.
Copyright © 2023 Taghavi, Zhao, Downham, Baena and Basu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- WHO guideline for screening and treatment of cervical precancer lesions for cervical cancer prevention. Available at: https://www.who.int/publications/i/item/9789240030824 (Accessed July 24, 2021).
-
- Mustafa RA, Santesso N, Khatib R, Mustafa AA, Wiercioch W, Kehar R, et al. Systematic reviews and meta-analyses of the accuracy of HPV tests, visual inspection with acetic acid, cytology, and colposcopy. Int J Gynecology Obstetrics (2016) 132(3):259–65. doi: 10.1016/j.ijgo.2015.07.024 - DOI - PubMed
-
- Arbyn M, Smith SB, Temin S, Sultana F, Castle P. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses on behalf of the Collaboration on Self-Sampling and HPV Testing. BMJ (2018) 363:4823. doi: 10.1136/bmj.k4823 - DOI - PMC - PubMed
-
- Zhao XL, Xu XQ, Duan XZ, Rezhake R, Hu SY, Wang Y, et al. Comparative performance evaluation of different HPV tests and triaging strategies using self-samples and feasibility assessment of thermal ablation in ‘colposcopy and treat’ approach: A population-based study in rural China. Int J Cancer (2020) 147(5):1275–85. doi: 10.1002/IJC.32881 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

