Cyclic Disulfide-Bridged Dinitroxide Biradical for Measuring Thiol Redox Status by Electron Paramagnetic Resonance
- PMID: 37811968
- PMCID: PMC10990597
- DOI: 10.1021/acs.jpcb.3c03387
Cyclic Disulfide-Bridged Dinitroxide Biradical for Measuring Thiol Redox Status by Electron Paramagnetic Resonance
Abstract
Among low-molecular-weight thiols, glutathione (GSH) is the main antioxidant in the cell, and its concentration is an indicator of the redox status. A cyclic disulfide-linked dinitroxide was designed for monitoring GSH by electron-paramagnetic resonance (EPR) spectroscopy. Reaction of the disulfide with GSH and three other thiols was measured at 9.6 GHz (X-band) and shown to be of first order in thiols. It is proposed that the reaction of the disulfide with 1 equiv of thiolate produced a short-lived intermediate that reacts with 1 equiv of thiolate to produce the cleavage product. The equilibrium ratio of the cleaved and intact disulfide is a measure of the redox state. Since the long-term goal is to use the disulfide to probe physiology in vivo, the feasibility of EPR spectroscopy and imaging of the disulfide and its cleavage product was demonstrated at 1 GHz (L-band).
Figures



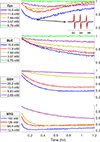
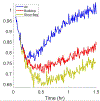

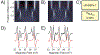
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

