Thrombocytopenia and platelet transfusions in ICU patients: an international inception cohort study (PLOT-ICU)
- PMID: 37812225
- PMCID: PMC10622358
- DOI: 10.1007/s00134-023-07225-2
Thrombocytopenia and platelet transfusions in ICU patients: an international inception cohort study (PLOT-ICU)
Erratum in
-
Correction: Thrombocytopenia and platelet transfusions in ICU patients: an international inception cohort study (PLOT-ICU).Intensive Care Med. 2024 Jan;50(1):154-155. doi: 10.1007/s00134-023-07291-6. Intensive Care Med. 2024. PMID: 38078947 Free PMC article. No abstract available.
Abstract
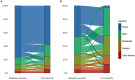
Purpose: Thrombocytopenia (platelet count < 150 × 109/L) is common in intensive care unit (ICU) patients and is likely associated with worse outcomes. In this study we present international contemporary data on thrombocytopenia in ICU patients.
Methods: We conducted a prospective cohort study in adult ICU patients in 52 ICUs across 10 countries. We assessed frequencies of thrombocytopenia, use of platelet transfusions and clinical outcomes including mortality. We evaluated pre-selected potential risk factors for the development of thrombocytopenia during ICU stay and associations between thrombocytopenia at ICU admission and 90-day mortality using pre-specified logistic regression analyses.
Results: We analysed 1166 ICU patients; the median age was 63 years and 39.5% were female. Overall, 43.2% (95% confidence interval (CI) 40.4-46.1) had thrombocytopenia; 23.4% (20-26) had thrombocytopenia at ICU admission, and 19.8% (17.6-22.2) developed thrombocytopenia during their ICU stay. Absence of acquired immune deficiency syndrome (AIDS), non-cancer-related immune deficiency, liver failure, male sex, septic shock, and bleeding at ICU admission were associated with the development of thrombocytopenia during ICU stay. Among patients with thrombocytopenia, 22.6% received platelet transfusion(s), and 64.3% of in-ICU transfusions were prophylactic. Patients with thrombocytopenia had higher occurrences of bleeding and death, fewer days alive without the use of life-support, and fewer days alive and out of hospital. Thrombocytopenia at ICU admission was associated with 90-day mortality (adjusted odds ratio 1.7; 95% CI 1.19-2.42).
Conclusion: Thrombocytopenia occurred in 43% of critically ill patients and was associated with worse outcomes including increased mortality. Platelet transfusions were given to 23% of patients with thrombocytopenia and most were prophylactic.
Keywords: Bleeding; Critical illness; Intensive care unit; Platelet transfusion; Thrombocytopenia; Thrombosis.
© 2023. The Author(s).
Conflict of interest statement
The Department of Intensive Care at Rigshospitalet (CTA, AG, MHM, AP, LR) has received funding for other projects from the Novo Nordisk Foundation, Sygeforsikringen ‘danmark’, and Pfizer and has conducted contract research for AM-Pharma. FP has received honoraria for consulting and lectures from Gilead and an institutional grant from Alexion Pharma. AP has received honoraria from Novartis for participation in an advisory board. EA has received research grants from MSD Avenir and Alexion and honoraria for lectures from Alexion, Sanofi and Pfizer. . AVDL has received honoraria from Sanofi for participation in an advisory board. PC has received consulting fees from Sanofi, Gilead, Alexion and Janssen and honoraria for lectures from Merck Sharp & Dohme, Gilead, Alexion and Pfizer. PP has received consulting fees from Sanofi and Gilead and honoraria from Merck Sharp & Dohme, Gilead, Mundipharma and Pfizer for academic and educational work. JH has received consulting fees from Paion and honoraria for lectures from the Finnish Medical Association, Laboratory Medicine and Duodecim. EC received fees for lectures and conference talks and had travel and accommodation expenses related to attending scientific meetings covered by Gilead, Shionogi B.V. and Sanofi-Genzyme. ARH has received honoraria from Pfizer for lectures. MHB has received consulting fees from AM-pharma and Inotrem. Full disclosure forms from all authors are available on the publisher’s website.
Figures