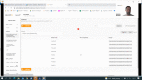
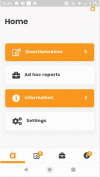
Development and usability testing of an electronic patient-reported outcome (ePRO) solution for patients with inflammatory diseases in an Advanced Therapy Medicinal Product (ATMP) basket trial
- PMID: 37812323
- PMCID: PMC10562321
- DOI: 10.1186/s41687-023-00634-3
Development and usability testing of an electronic patient-reported outcome (ePRO) solution for patients with inflammatory diseases in an Advanced Therapy Medicinal Product (ATMP) basket trial
Abstract
Background: Electronic patient-reported outcome (ePRO) systems are increasingly used in clinical trials to provide evidence of efficacy and tolerability of treatment from the patient perspective. The aim of this study is twofold: (1) to describe how we developed an electronic platform for patients to report their symptoms, and (2) to develop and undertake usability testing of an ePRO solution for use in a study of cell therapy seeking to provide early evidence of efficacy and tolerability of treatment and test the feasibility of the system for use in later phase studies.
Methods: An ePRO system was designed to be used in a single arm, multi-centre, phase II basket trial investigating the safety and activity of the use of ORBCEL-C™ in the treatment of patients with inflammatory conditions. ORBCEL-C™ is an enriched Mesenchymal Stromal Cells product isolated from human umbilical cord tissue using CD362+ cell selection. Usability testing sessions were conducted using cognitive interviews and the 'Think Aloud' method with patient advisory group members and Research Nurses to assess the usability of the system.
Results: Nine patient partners and seven research nurses took part in one usability testing session. Measures of fatigue and health-related quality of life, the PRO-CTCAE™ and FACT-GP5 global tolerability question were included in the ePRO system. Alert notifications to the clinical team were triggered by PRO-CTCAE™ and FACT-GP5 scores. Patient participants liked the simplicity and responsiveness of the patient-facing app. Two patients were unable to complete the testing session, due to technical issues. Research Nurses suggested minor modifications to improve functionality and the layout of the clinician dashboard and the training materials.
Conclusion: By testing the effectiveness, efficiency, and satisfaction of our novel ePRO system (PROmicsR), we learnt that most people with an inflammatory condition found it easy to report their symptoms using an app on their own device. Their experiences using the PROmicsR ePRO system within a trial environment will be further explored in our upcoming feasibility testing. Research nurses were also positive and found the clinical dashboard easy-to-use. Using ePROs in early phase trials is important in order to provide evidence of therapeutic responses and tolerability, increase the evidence based, and inform methodology development.
Trial registration: ISRCTN, ISRCTN80103507. Registered 01 April 2022, https://www.isrctn.com/ISRCTN80103507.
Keywords: Cognitive interviews; Early phase advanced therapy trial; Electronic patient reported outcomes; Inflammatory conditions; Usability testing.
Plain language summary
More and more patients tell clinicians how they feel by completing questionnaires electronically. Therefore, it is important to assess how easy it is for patients to do this. In this study, we describe how we developed an electronic platform for patients to report their symptoms and how we tested the usability of this platform with patient partners and research nurses. Once the electronic platform was developed, quality of life and symptoms questionnaires were programmed onto it. Alerts were sent to the clinical team if specific scores were obtained on the symptoms questionnaires. Although two patient partners were not able to finish the testing session because of technical issues, the ones who completed the session liked its simplicity and responsiveness. The research nurses also liked the system and only suggested minor modifications. Following this testing, we refined the electronic platform to test it further in a larger study which investigates the safety and use of a drug. We hope that thanks to this electronic platform, we will obtain useful information on the safety and efficacy of treatment.
© 2023. International Society for Quality of Life Research (ISOQOL).
Conflict of interest statement
CM receives funding from NIHR Surgical Reconstruction and Microbiology Research Centre (SRMRC), UKRI, and declares personal fees from Aparito Ltd outside the submitted work. SEH is supported by the National Institute for Health Research (NIHR) Applied Research Centre (ARC) West Midlands at the University of Birmingham and UKRI. SEH has received personal fees from Cochlear Ltd and Aparito Ltd outside the submitted work. OLA receives funding from the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre, NIHR ARC West Midlands at the University of Birmingham, the Brain Tumour Charity, Innovate UK (part of UK Research and Innovation), Gilead Sciences Ltd. and Janssen Pharmaceuticals Inc. OLA declares personal fees from Gilead Sciences Ltd., GlaxoSmithKline (GSK) and Merck outside the submitted work. MC is a National Institute for Health Research (NIHR) Senior Investigator and receives funding from the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre, the NIHR Surgical Reconstruction and Microbiology Research Centre and NIHR ARC West Midlands at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Health Data Research UK, UKRI, Macmillan Cancer Support, UCB Pharma, Gilead, Janssen and GSK. MC has received personal fees from Astellas, Aparito Ltd, CIS Oncology, Takeda, Merck, Daiichi Sankyo, Glaukos, GSK and the Patient-Centred Outcomes Research Institute (PCORI) outside the submitted work. DK reports grants from Macmillan Cancer Support, the NIHR, NIHR Birmingham Biomedical Research Centre, and NIHR SRMRC at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, and personal fees from Merck and GSK outside the submitted work.
Figures
Similar articles
-
Testing an Electronic Patient-Reported Outcome Platform in the Context of Traumatic Brain Injury: PRiORiTy Usability Study.JMIR Form Res. 2025 Jan 23;9:e58128. doi: 10.2196/58128. JMIR Form Res. 2025. PMID: 39864101 Free PMC article.
-
Feasibility of a new electronic patient-reported outcome (ePRO) system for an advanced therapy clinical trial in immune-mediated inflammatory disease (PROmics): protocol for a qualitative feasibility study.BMJ Open. 2022 Sep 6;12(9):e063199. doi: 10.1136/bmjopen-2022-063199. BMJ Open. 2022. PMID: 36691123 Free PMC article.
-
Recommendations on evidence needed to support measurement equivalence between electronic and paper-based patient-reported outcome (PRO) measures: ISPOR ePRO Good Research Practices Task Force report.Value Health. 2009 Jun;12(4):419-29. doi: 10.1111/j.1524-4733.2008.00470.x. Epub 2008 Nov 11. Value Health. 2009. PMID: 19900250
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Key methodological considerations for usability testing of electronic patient-reported outcome (ePRO) systems.Qual Life Res. 2020 Feb;29(2):325-333. doi: 10.1007/s11136-019-02329-z. Epub 2019 Oct 18. Qual Life Res. 2020. PMID: 31691202 Free PMC article. Review.
Cited by
-
Challenges and Opportunities in Developing Tailored Pain Management Strategies for Liver Patients.Cureus. 2023 Dec 16;15(12):e50633. doi: 10.7759/cureus.50633. eCollection 2023 Dec. Cureus. 2023. PMID: 38226103 Free PMC article. Review.
-
Advancing patient-centric care: integrating patient reported outcomes for tolerability assessment in early phase clinical trials - insights from an expert virtual roundtable.EClinicalMedicine. 2024 Sep 24;76:102838. doi: 10.1016/j.eclinm.2024.102838. eCollection 2024 Oct. EClinicalMedicine. 2024. PMID: 39386161 Free PMC article. Review.
-
Measure selection for an electronic patient-reported outcome (ePRO) system for CAR T-cell therapy patients: a modified Delphi consensus study.EClinicalMedicine. 2025 May 28;84:103256. doi: 10.1016/j.eclinm.2025.103256. eCollection 2025 Jun. EClinicalMedicine. 2025. PMID: 40510921 Free PMC article.
-
Testing an Electronic Patient-Reported Outcome Platform in the Context of Traumatic Brain Injury: PRiORiTy Usability Study.JMIR Form Res. 2025 Jan 23;9:e58128. doi: 10.2196/58128. JMIR Form Res. 2025. PMID: 39864101 Free PMC article.
References
-
- Agency EM (2008) Guideline on safety and efficacy follow-up—risk management of advanced therapy medicinal products. London
-
- FDA, Patient-reported outcome measures: use in medicinal product development to support labelling claims. 2009.
-
- Fairclough D. Design and analysis of quality of life studies in clinical trials. Boca Raton: Chapman & Hall/CRC Press; 2002.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous