Efficacy and Safety of XEN1101, a Novel Potassium Channel Opener, in Adults With Focal Epilepsy: A Phase 2b Randomized Clinical Trial
- PMID: 37812429
- PMCID: PMC10562989
- DOI: 10.1001/jamaneurol.2023.3542
Efficacy and Safety of XEN1101, a Novel Potassium Channel Opener, in Adults With Focal Epilepsy: A Phase 2b Randomized Clinical Trial
Erratum in
-
Addition of Nonauthor Collaborators and a Supplement.JAMA Neurol. 2024 Feb 1;81(2):200. doi: 10.1001/jamaneurol.2023.5238. JAMA Neurol. 2024. PMID: 38165709 Free PMC article. No abstract available.
Abstract
Importance: Many patients with focal epilepsy experience seizures despite treatment with currently available antiseizure medications (ASMs) and may benefit from novel therapeutics.
Objective: To evaluate the efficacy and safety of XEN1101, a novel small-molecule selective Kv7.2/Kv7.3 potassium channel opener, in the treatment of focal-onset seizures (FOSs).
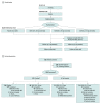
Design, setting, and participants: This phase 2b, randomized, double-blind, placebo-controlled, parallel-group, dose-ranging adjunctive trial investigated XEN1101 over an 8-week treatment period from January 30, 2019, to September 2, 2021, and included a 6-week safety follow-up. Adults experiencing 4 or more monthly FOSs while receiving stable treatment (1-3 ASMs) were enrolled at 97 sites in North America and Europe.
Interventions: Patients were randomized 2:1:1:2 to receive XEN1101, 25, 20, or 10 mg, or placebo with food once daily for 8 weeks. Dosage titration was not used. On completion of the double-blind phase, patients were offered the option of entering an open-label extension (OLE). Patients not participating in the OLE had follow-up safety visits (1 and 6 weeks after the final dose).
Main outcomes and measures: The primary efficacy end point was the median percent change from baseline in monthly FOS frequency. Treatment-emergent adverse events (TEAEs) were recorded and comprehensive laboratory assessments were made. Modified intention-to-treat analysis was conducted.
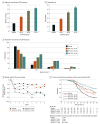
Results: A total of 325 patients who were randomized and treated were included in the safety analysis; 285 completed the 8-week double-blind phase. In the 325 patients included, mean (SD) age was 40.8 (13.3) years, 168 (51.7%) were female, and 298 (91.7%) identified their race as White. Treatment with XEN1101 was associated with seizure reduction in a robust dose-response manner. The median (IQR) percent reduction from baseline in monthly FOS frequency was 52.8% (P < .001 vs placebo; IQR, -80.4% to -16.9%) for 25 mg, 46.4% (P < .001 vs placebo; IQR, -76.7% to -14.0%) for 20 mg, and 33.2% (P = .04 vs placebo; IQR, -61.8% to 0.0%) for 10 mg, compared with 18.2% (IQR, -37.3% to 7.0%) for placebo. XEN1101 was generally well tolerated and TEAEs were similar to those of commonly prescribed ASMs, and no TEAEs leading to death were reported.
Conclusions and relevance: The efficacy and safety findings of this clinical trial support the further clinical development of XEN1101 for the treatment of FOSs.
Trial registration: ClinicalTrials.gov Identifier: NCT03796962.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

