Tau Oligomer-Containing Synapse Elimination by Microglia and Astrocytes in Alzheimer Disease
- PMID: 37812432
- PMCID: PMC10562992
- DOI: 10.1001/jamaneurol.2023.3530
Tau Oligomer-Containing Synapse Elimination by Microglia and Astrocytes in Alzheimer Disease
Abstract
Importance: Factors associated with synapse loss beyond amyloid-β plaques and neurofibrillary tangles may more closely correlate with the emergence of cognitive deficits in Alzheimer disease (AD) and be relevant for early therapeutic intervention.
Objective: To investigate whether accumulation of tau oligomers in synapses is associated with excessive synapse elimination by microglia or astrocytes and with cognitive outcomes (dementia vs no dementia [hereinafter termed resilient]) of individuals with equal burdens of AD neuropathologic changes at autopsy.
Design, setting, and participants: This cross-sectional postmortem study included 40 human brains from the Massachusetts Alzheimer Disease Research Center Brain Bank with Braak III to IV stages of tau pathology but divergent antemortem cognition (dementia vs resilient) and cognitively normal controls with negligible AD neuropathologic changes. The visual cortex, a region without tau tangle deposition at Braak III to IV stages, was assessed after expansion microscopy to analyze spatial relationships of synapses with microglia and astrocytes. Participants were matched for age, sex, and apolipoprotein E status. Evidence of Lewy bodies, TDP-43 aggregates, or other lesions different from AD neuropathology were exclusion criteria. Tissue was collected from July 1998 to November 2020, and analyses were conducted from February 1, 2022, through May 31, 2023.
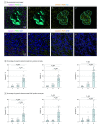
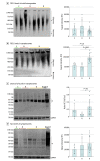
Main outcomes and measures: Amyloid-β plaques, tau neuropil thread burden, synapse density, tau oligomers in synapses, and internalization of tau oligomer-tagged synapses by microglia and astrocytes were quantitated. Analyses were performed using 1-way analysis of variance for parametric variables and the Kruskal-Wallis test for nonparametric variables; between-group differences were evaluated with Holm-Šídák tests.
Results: Of 40 included participants (mean [SD] age at death, 88 [8] years; 21 [52%] male), 19 had early-stage dementia with Braak stages III to IV, 13 had resilient brains with similar Braak stages III to IV, and 8 had no dementia (Braak stages 0-II). Brains with dementia but not resilient brains had substantial loss of presynaptic (43%), postsynaptic (33%), and colocalized mature synaptic elements (38%) compared with controls and significantly higher percentages of mature synapses internalized by IBA1-positive microglia (mean [SD], 13.3% [3.9%] in dementia vs 2.6% [1.9%] in resilient vs 0.9% [0.5%] in control; P < .001) and by GFAP-positive astrocytes (mean [SD], 17.2% [10.9%] in dementia vs 3.7% [4.0%] in resilient vs 2.7% [1.8%] in control; P = .001). In brains with dementia but not in resilient brains, tau oligomers more often colocalized with synapses, and the proportions of tau oligomer-containing synapses inside microglia (mean [SD] for presynapses, mean [SD], 7.4% [1.8%] in dementia vs 5.1% [1.9%] resilient vs 3.7% [0.8%] control; P = .006; and for postsynapses 11.6% [3.6%] dementia vs 6.8% [1.3%] resilient vs 7.4% [2.5%] control; P = .001) and astrocytes (mean [SD] for presynapses, 7.0% [2.1%] dementia vs 4.3% [2.2%] resilient vs 4.0% [0.7%] control; P = .001; and for postsynapses, 7.9% [2.2%] dementia vs 5.3% [1.8%] resilient vs 3.0% [1.5%] control; P < .001) were significantly increased compared with controls. Those changes in brains with dementia occurred in the absence of tau tangle deposition in visual cortex.
Conclusion and relevance: The findings from this cross-sectional study suggest that microglia and astrocytes may excessively engulf synapses in brains of individuals with dementia and that the abnormal presence of tau oligomers in synapses may serve as signals for increased glial-mediated synapse elimination and early loss of brain function in AD.
Conflict of interest statement
Figures


Comment in
-
Synaptic Oligomers and Glial Cells in Alzheimer Disease.JAMA Neurol. 2023 Nov 1;80(11):1136-1137. doi: 10.1001/jamaneurol.2023.3539. JAMA Neurol. 2023. PMID: 37812438 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

