Normal and Sjogren's syndrome models of the murine lacrimal gland studied at single-cell resolution
- PMID: 37812717
- PMCID: PMC10589653
- DOI: 10.1073/pnas.2311983120
Normal and Sjogren's syndrome models of the murine lacrimal gland studied at single-cell resolution
Abstract
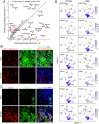
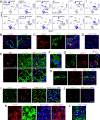
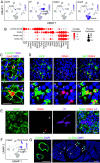
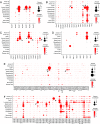
The lacrimal gland is of central interest in ophthalmology both as the source of the aqueous component of tear fluid and as the site of autoimmune pathology in the context of Sjogren's syndrome (SjS). To provide a foundational description of mouse lacrimal gland cell types and their patterns of gene expression, we have analyzed single-cell transcriptomes from wild-type (Balb/c) mice and from two genetically based SjS models, MRL/lpr and NOD (nonobese diabetic).H2b, and defined the localization of multiple cell-type-specific protein and mRNA markers. This analysis has uncovered a previously undescribed cell type, Car6+ cells, which are located at the junction of the acini and the connecting ducts. More than a dozen secreted polypeptides that are likely to be components of tear fluid are expressed by acinar cells and show pronounced sex differences in expression. Additional examples of gene expression heterogeneity within a single cell type were identified, including a gradient of Claudin4 along the length of the ductal system and cell-to-cell heterogeneity in transcription factor expression within acinar and myoepithelial cells. The patterns of expression of channels, transporters, and pumps in acinar, Car6+, and ductal cells make strong predictions regarding the mechanisms of water and electrolyte secretion. In MRL/lpr and NOD.H2b lacrimal glands, distinctive changes in parenchymal gene expression and in immune cell subsets reveal widespread interferon responses, a T cell-dominated infiltrate in the MRL/lpr model, and a mixed B cell and T cell infiltrate in the NOD.H2b model.
Keywords: autoimmunity; exocrine gland; lacrimal gland; ophthalmology; scRNAseq.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Tsubota K., Tear dynamics and dry eye. Prog. Retin. Eye Res. 17, 565–596 (1998). - PubMed
-
- Tiffany J. M., The normal tear film. Dev. Ophthalmol. 41, 1–20 (2008). - PubMed
-
- Schechter J. E., Warren D. W., Mircheff A. K., A lacrimal gland is a lacrimal gland, but rodent’s and rabbit’s are not human. Ocul. Surf. 8, 111–134 (2010). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

