Direct neuronal conversion of microglia/macrophages reinstates neurological function after stroke
- PMID: 37812721
- PMCID: PMC10589698
- DOI: 10.1073/pnas.2307972120
Direct neuronal conversion of microglia/macrophages reinstates neurological function after stroke
Abstract
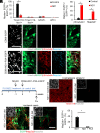
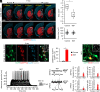
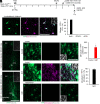
Although generating new neurons in the ischemic injured brain would be an ideal approach to replenish the lost neurons for repairing the damage, the adult mammalian brain retains only limited neurogenic capability. Here, we show that direct conversion of microglia/macrophages into neurons in the brain has great potential as a therapeutic strategy for ischemic brain injury. After transient middle cerebral artery occlusion in adult mice, microglia/macrophages converge at the lesion core of the striatum, where neuronal loss is prominent. Targeted expression of a neurogenic transcription factor, NeuroD1, in microglia/macrophages in the injured striatum enables their conversion into induced neuronal cells that functionally integrate into the existing neuronal circuits. Furthermore, NeuroD1-mediated induced neuronal cell generation significantly improves neurological function in the mouse stroke model, and ablation of these cells abolishes the gained functional recovery. Our findings thus demonstrate that neuronal conversion contributes directly to functional recovery after stroke.
Keywords: NeuroD1; direct reprogramming; microglia; stroke.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

