Ultrasound-assisted continuous crystallization of metastable polymorphic pharmaceutical in a slug-flow tubular crystallizer
- PMID: 37813044
- PMCID: PMC10568301
- DOI: 10.1016/j.ultsonch.2023.106627
Ultrasound-assisted continuous crystallization of metastable polymorphic pharmaceutical in a slug-flow tubular crystallizer
Abstract
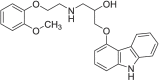
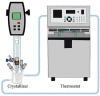
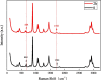
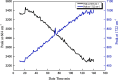
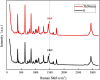
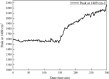
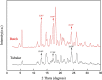
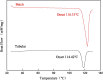
Metastable polymorphic pharmaceuticals have garnered significant attention in recent years due to their enhanced physicochemical properties, including solubility, bioavailability, and intellectual property considerations. However, the manufacturing of metastable form pharmaceuticals remains a formidable challenge. The stable preparation of metastable carvedilol (CVD) form Ⅱ crystals during CVD production is elusive, leading to substantial inconsistencies in product quality and regulatory compliance. In this study, we successfully prepared metastable CVD Form Ⅱ crystals using a continuous tubular crystallizer. Our findings demonstrate that the tubular crystallizer exhibits high efficiency and robustness for generating metastable crystal Form Ⅱ. We optimized the crystallization process by incorporating air bubble segments and employing ultrasonic irradiation strategies to overcome blockages and wall sticking issues encountered during operation. Ultimately, we developed an ultrasound-assisted continuous slug-flow tubular crystallization method and evaluated its performance. The results indicate that the CVD crystals produced through this process are resilient, sustainable, and uninterrupted products with promising potential for producing metastable polymorphic pharmaceuticals while effectively addressing encrustation problems associated with continuous tubular crystallization.
Keywords: Carvedilol; Polymorphic transformation; Sonocrystallization; Tubular crystallizer; Ultrasonic irradiation.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Cao Y., Zhang K., Gao Z., Wang J., Rohani S., Gong J. Preparation, Stabilization, and Dissolution Enhancement of Vortioxetine Hydrobromide Metastable Polymorphs in Silica Nanopores. Cryst. Growth Des. 2021;22(1):191–199.
-
- Woollam G.R., Das P.P., Mugnaioli E., Andrusenko I., Galanis A.S., van de Streek J., Nicolopoulos S., Gemmi M., Wagner T. Structural analysis of metastable pharmaceutical loratadine form II, by 3D electron diffraction and DFT+D energy minimisation. CrstEngComm. 2020;22(43):7490–7499.
-
- Agnew L.R., McGlone T., Wheatcroft H.P., Robertson A., Parsons A.R., Wilson C.C. Continuous Crystallization of Paracetamol (Acetaminophen) Form II: Selective Access to a Metastable Solid Form. Cryst. Growth Des. 2017;17(5):2418–2427.
-
- Liu Y., Yan H., Yang J., Yao M., Yu C., Yin H., Chen M., Gong J. Particle design of the metastable form of clopidogrel hydrogen sulfate by building spherulitic growth operating spaces in binary solvent systems. Powder Technol. 2021;386:70–80.
-
- Fang L., Gao Z., Gao Z., Huang W., Wan X., Rohani S., Gong J. Controlled crystallization of metastable polymorphic pharmaceutical: Comparative study of batchwise and continuous tubular crystallizers. Chem. Eng. Sci. 2023;266:118277.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

