Comprehensive review on the pathogenesis of hypertriglyceridaemia-associated acute pancreatitis
- PMID: 37813108
- PMCID: PMC10563627
- DOI: 10.1080/07853890.2023.2265939
Comprehensive review on the pathogenesis of hypertriglyceridaemia-associated acute pancreatitis
Abstract
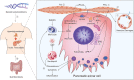
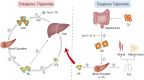
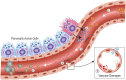
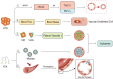
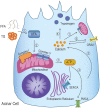
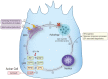
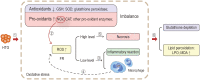
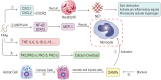
It is well known, that the inflammatory process that characterizes acute pancreatitis (AP) can lead to both pancreatic damage and systemic inflammatory response syndrome (SIRS). During the last 20 years, there has been a growing incidence of episodes of acute pancreatitis associated with hypertriglyceridaemia (HTAP). This review provides an overview of triglyceride metabolism and the potential mechanisms that may contribute to developing or exacerbating HTAP. The article comprehensively discusses the various pathological roles of free fatty acid, inflammatory response mechanisms, the involvement of microcirculation, serum calcium overload, oxidative stress and the endoplasmic reticulum, genetic polymorphism, and gut microbiota, which are known to trigger or escalate this condition. Future perspectives on HTAP appear promising, with ongoing research focused on developing more specific and effective treatment strategies.
Keywords: Hypertriglyceridemia; acute pancreatitis; lipids; mechanism; pathogenesis.
Conflict of interest statement
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author upon request.
Ethics statement
This study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University. This study was performed according to the principles expressed in the Declaration of Helsinki. The committee decided to waive the need for written informed consent from the participants studied in this analysis as the data were analyzed retrospectively and anonymously.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
