The OSCAR-MP Consensus Criteria for Quality Assessment of Retinal Optical Coherence Tomography Angiography
- PMID: 37813596
- PMCID: PMC10574825
- DOI: 10.1212/NXI.0000000000200169
The OSCAR-MP Consensus Criteria for Quality Assessment of Retinal Optical Coherence Tomography Angiography
Abstract
Background and objectives: Optical coherence tomography angiography (OCTA) is a noninvasive high-resolution imaging technique for assessing the retinal vasculature and is increasingly used in various ophthalmologic, neuro-ophthalmologic, and neurologic diseases. To date, there are no validated consensus criteria for quality control (QC) of OCTA. Our study aimed to develop criteria for OCTA quality assessment.
Methods: To establish criteria through (1) extensive literature review on OCTA artifacts and image quality to generate standardized and easy-to-apply OCTA QC criteria, (2) application of OCTA QC criteria to evaluate interrater agreement, (3) identification of reasons for interrater disagreement, revision of OCTA QC criteria, development of OCTA QC scoring guide and training set, and (4) validation of QC criteria in an international, interdisciplinary multicenter study.
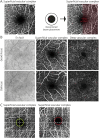
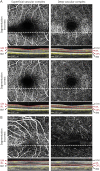
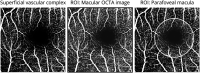
Results: We identified 7 major aspects that affect OCTA quality: (O) obvious problems, (S) signal strength, (C) centration, (A) algorithm failure, (R) retinal pathology, (M) motion artifacts, and (P) projection artifacts. Seven independent raters applied the OSCAR-MP criteria to a set of 40 OCTA scans from people with MS, Sjogren syndrome, and uveitis and healthy individuals. The interrater kappa was substantial (κ 0.67). Projection artifacts were the main reason for interrater disagreement. Because artifacts can affect only parts of OCTA images, we agreed that prior definition of a specific region of interest (ROI) is crucial for subsequent OCTA quality assessment. To enhance artifact recognition and interrater agreement on reduced image quality, we designed a scoring guide and OCTA training set. Using these educational tools, 23 raters from 14 different centers reached an almost perfect agreement (κ 0.92) for the rejection of poor-quality OCTA images using the OSCAR-MP criteria.
Discussion: We propose a 3-step approach for standardized quality control: (1) To define a specific ROI, (2) to assess the occurrence of OCTA artifacts according to the OSCAR-MP criteria, and (3) to evaluate OCTA quality based on the occurrence of different artifacts within the ROI. OSCAR-MP OCTA QC criteria achieved high interrater agreement in an international multicenter study and is a promising QC protocol for application in the context of future clinical trials and studies.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
R. Wicklein received a poster grant from Novartis; C. Yam reports no conflict of interest; C. Noll reports no disclosures relevant to the manuscript; L. Aly received travel and research support by Novartis; N. Banze reports no disclosures relevant to the manuscript; E.F. Romahn reports no disclosures relevant to the manuscript; E. Wolf reports no disclosures relevant to the manuscript; B. Hemmer has served on scientific advisory boards for Novartis; he has served as DMSC member for AllergyCare, Sandoz, Polpharma, Biocon, and TG therapeutics; his institution received research grants from Roche for multiple sclerosis research. He has received honoraria for counseling (Gerson Lehrmann Group). He holds part of 2 patents; one for the detection of antibodies against KIR4.1 in a subpopulation of patients with multiple sclerosis and one for genetic determinants of neutralizing antibodies to interferon. All conflicts are not relevant to the topic of the study. He is associated with DIFUTURE (Data Integration for Future Medicine) [BMBF 01ZZ1804[A-I]]; F.C. Oertel reports no disclosures relevant to the manuscript; H.G. Zimmermann received research grants and speaking honoraria from Novartis; P. Albrecht received, with approval of the Rector of Heinrich-Heine University and the CEO of University of Düsseldorf Hospital, personal fees, research grants, and nonfinancial support from Allergan, Biogen, Celgene, Janssen Cilag, Ipsen, Merck Serono, Merz Pharmaceuticals, Novartis, and Roche, personal fees and nonfinancial support from Bayer Healthcare, Teva, and Sanofi-Aventis/Genzyme, grants from the German Research Foundation (DFG) all outside the submitted work; M. Ringelstein received speaker honoraria from Novartis, Bayer Vital GmbH, Roche, Alexion, Horizon, and Ipsen and travel reimbursement from Bayer Schering, Biogen Idec, Merz, Genzyme, Teva, Roche, Horizon, and Merck, none related to this study; C. Baumann reports no disclosures relevant to the manuscript; N. Feucht reports no disclosures relevant to the manuscript; J. Penkava reports no disclosures relevant to the manuscript; J. Havla reports personal fees and nonfinancial support from Alexion, Horizon, Roche, Merck, Novartis, Biogen, BMS, and Janssen and nonfinancial support from the Guthy-Jackson Charitable Foundation and the Sumaira Foundation; J.A. Gernert received travel expenses and nonfinancial support from Merck; C. Mardin is a medical advisor to Heidelberg Engineering, Heidelberg, Germany, receives lecture honorarium by Heidelberg Engineering, Bayer AG, Leverkusen, Germany, and is partially funded by Federal Ministry of Education and Research and Bavarian Ministry of Health; E. Vasileiou reports no disclosures relevant to the manuscript; A. van der Walt served on advisory boards for Novartis, Biogen, Merck, Roche, and NervGen. She received unrestricted research grants from Novartis, Biogen, Merck, and Roche. She is currently a coprincipal investigator on a cosponsored observational study with Roche, evaluating a Roche-developed smartphone app, Floodlight-MS. She has received speaker's honoraria and travel support from Novartis, Roche, Biogen, and Merck. She serves as the Chief operating Officer of the MSBase Foundation (not for profit). Her primary research support is from the National Health and Medical Research Council of Australia and MS Research Australia; O. Al-Louzi has received grant support from the National Multiple Sclerosis Society and American Brain Foundation (FAN-1807-32163) unrelated to the current project; S. Cabello reports no disclosures relevant to the manuscript; A. Vidal-Jordana has engaged in consulting and/or participated as speaker in events organized by Roche, Novartis, Merck, and Sanofi, none of them related to this work; J. Krämer received honoraria for lecturing from Biogen, Novartis, Sanofi Genzyme, Roche, Mylan, and Teva and financial research support from Sanofi Genzyme, Novartis, Roche, and Amicus Therapeutics; H. Wiendl received compensation for serving on Scientific Advisory Boards/Steering Committees for Bayer Healthcare, Biogen Idec, Sanofi Genzyme, Merck Serono, and Novartis. He has received speaker honoraria and travel support from Bayer Vital GmbH, Bayer Schering AG, Biogen, CSL Behring, EMD Serono, Fresenius Medical Care, Genzyme, Merck Serono, Omniamed, Novartis, and Sanofi Aventis. He has received compensation as a consultant from Biogen Idec, Merck Serono, Novartis, Roche, and Sanofi Genzyme. H. Wiendl also received research support from Bayer Healthcare, Bayer Vital, Biogen Idec, Merck Serono, Novartis, Sanofi Genzyme, Sanofi US, and Teva; J. Lizrova Preiningerova received travel support and speaking honoraria from Roche, Genzyme, Novartis, and Biogen and received a research grant from Biogen; O. Ciccarelli is a member of independent DSMB for Novartis, gave a teaching talk on McDonald criteria in a Merck local symposium, and contributed to an Advisory Board for Biogen; she is Deputy Editor of
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
