Multivalent insulin receptor activation using insulin-DNA origami nanostructures
- PMID: 37813939
- PMCID: PMC10873203
- DOI: 10.1038/s41565-023-01507-y
Multivalent insulin receptor activation using insulin-DNA origami nanostructures
Abstract
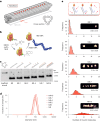
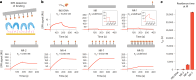
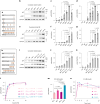
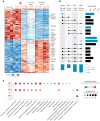
Insulin binds the insulin receptor (IR) and regulates anabolic processes in target tissues. Impaired IR signalling is associated with multiple diseases, including diabetes, cancer and neurodegenerative disorders. IRs have been reported to form nanoclusters at the cell membrane in several cell types, even in the absence of insulin binding. Here we exploit the nanoscale spatial organization of the IR to achieve controlled multivalent receptor activation. To control insulin nanoscale spatial organization and valency, we developed rod-like insulin-DNA origami nanostructures carrying different numbers of insulin molecules with defined spacings. Increasing the insulin valency per nanostructure markedly extended the residence time of insulin-DNA origami nanostructures at the receptors. Both insulin valency and spacing affected the levels of IR activation in adipocytes. Moreover, the multivalent insulin design associated with the highest levels of IR activation also induced insulin-mediated transcriptional responses more effectively than the corresponding monovalent insulin nanostructures. In an in vivo zebrafish model of diabetes, treatment with multivalent-but not monovalent-insulin nanostructures elicited a reduction in glucose levels. Our results show that the control of insulin multivalency and spatial organization with nanoscale precision modulates the IR responses, independent of the insulin concentration. Therefore, we propose insulin nanoscale organization as a design parameter in developing new insulin therapies.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

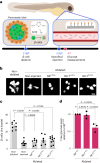






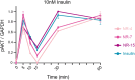
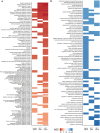
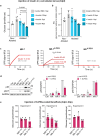
References
-
- James DE, Stöckli J, Birnbaum MJ. The aetiology and molecular landscape of insulin resistance. Nat. Rev. Mol. Cell Biol. 2021;22:751–771. - PubMed
MeSH terms
Substances
Grants and funding
- NNF19OC0057238/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20OC0063993/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF22OC0075245/Novo Nordisk Fonden (Novo Nordisk Foundation)
- DIA2021-684/Diabetesfonden (Stiftelsen Svenska Diabetesförbundets Forskningsfond)
- NA/European Foundation for the Study of Diabetes (EFSD)
- ERC CoG 617711/EC | EC Seventh Framework Programm | FP7 Ideas: European Research Council (FP7-IDEAS-ERC - Specific Programme: "Ideas" Implementing the Seventh Framework Programme of the European Community for Research, Technological Development and Demonstration Activities (2007 to 2013))
- 2020-01856/Vetenskapsrådet (Swedish Research Council)
- 2016-00786/Vetenskapsrådet (Swedish Research Council)
- 2022-02743/Vetenskapsrådet (Swedish Research Council)
- KAW 2017.0114/Knut och Alice Wallenbergs Stiftelse (Knut and Alice Wallenberg Foundation)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

