Glutamine mimicry suppresses tumor progression through asparagine metabolism in pancreatic ductal adenocarcinoma
- PMID: 37814011
- PMCID: PMC10956382
- DOI: 10.1038/s43018-023-00649-1
Glutamine mimicry suppresses tumor progression through asparagine metabolism in pancreatic ductal adenocarcinoma
Abstract
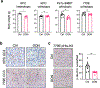
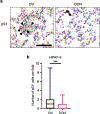
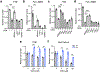
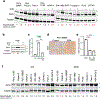
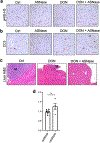
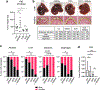
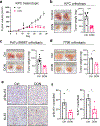
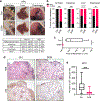
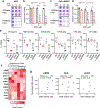
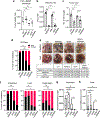
In pancreatic ductal adenocarcinoma (PDAC), glutamine is a critical nutrient that drives a wide array of metabolic and biosynthetic processes that support tumor growth. Here, we elucidate how 6-diazo-5-oxo-L-norleucine (DON), a glutamine antagonist that broadly inhibits glutamine metabolism, blocks PDAC tumor growth and metastasis. We find that DON significantly reduces asparagine production by inhibiting asparagine synthetase (ASNS), and that the effects of DON are rescued by asparagine. As a metabolic adaptation, PDAC cells upregulate ASNS expression in response to DON, and we show that ASNS levels are inversely correlated with DON efficacy. We also show that L-asparaginase (ASNase) synergizes with DON to affect the viability of PDAC cells, and that DON and ASNase combination therapy has a significant impact on metastasis. These results shed light on the mechanisms that drive the effects of glutamine mimicry and point to the utility of cotargeting adaptive responses to control PDAC progression.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

