Clinical effectiveness of routine first-trimester combined screening for pre-eclampsia in Spain with the addition of placental growth factor
- PMID: 37814344
- PMCID: PMC10619612
- DOI: 10.1111/aogs.14687
Clinical effectiveness of routine first-trimester combined screening for pre-eclampsia in Spain with the addition of placental growth factor
Abstract
Introduction: Pre-eclampsia affects 2%-8% of pregnancies and is one of the leading causes of maternal and perinatal morbidity and mortality. First-trimester screening using an algorithm that combines maternal characteristics, mean arterial blood pressure, uterine artery pulsatility index and biomarkers (pregnancy-associated plasma protein-A and placental growth factor) is the method that achieves a greater diagnostic accuracy. It has been shown that daily salicylic acid administration before 16 weeks in women at a high risk for pre-eclampsia can reduce the incidence of preterm pre-eclampsia. However, no previous studies have evaluated the impact of routine first-trimester combined screening for pre-eclampsia with placental growth factor after being implemented in the clinical practice.
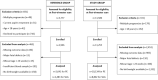
Material and methods: This was a multicenter cohort study conducted in eight different maternities across Spain. Participants in the reference group were prospectively recruited between October 2015 and September 2017. Participants in the study group were retrospectively recruited between March 2019 and May 2021. Pre-eclampsia risk was calculated between 11+0 and 13+6 weeks using the Gaussian algorithm combining maternal characteristics, mean arterial pressure, uterine arteries pulsatility index, pregnancy-associated plasma protein-A and placental growth factor. Patients with a risk greater than 1/170 were prescribed daily salicylic acid 150 mg until 36 weeks. Patients in the reference group did not receive salicylic acid during gestation.
Results: A significant reduction was observed in preterm pre-eclampsia (OR 0.47; 95% CI: 0.30-0.73), early-onset (<34 weeks) pre-eclampsia (OR 0.35; 95% CI: 0.16-0.77), preterm small for gestational age newborn (OR 0.57; 95% CI: 0.40-0.82), spontaneous preterm birth (OR 0.72; 95% CI: 0.57-0.90), and admission to intensive care unit (OR 0.55; 95% CI: 0.37-0.81). A greater treatment adherence resulted in a significant reduction in adverse outcomes.
Conclusions: Routine first-trimester screening for pre-eclampsia with placental growth factor leads to a reduction in preterm pre-eclampsia and other pregnancy complications. Aspirin treatment compliance has a great impact on the effectiveness of this screening program.
Keywords: PlGF; aspirin; placental growth factor; pre-eclampsia; salicylic acid; screening.
© 2023 The Authors. Acta Obstetricia et Gynecologica Scandinavica published by John Wiley & Sons Ltd on behalf of Nordic Federation of Societies of Obstetrics and Gynecology (NFOG).
Conflict of interest statement
MM received lecture fees from Roche Diagnostics. The remaining authors report no conflict of interest.
Figures

References
-
- Duley L. The global impact of pre‐eclampsia and eclampsia. Semin Perinatol. 2009;33:130‐137. - PubMed
-
- Tang LC, Kwok AC, Wong AY, Lee YY, Sun KO, So AP. Critical care in obstetrical patients: an eight‐year review. Chin Med J (Engl). 1997;110:936‐941. - PubMed
-
- Tan MY, Wright D, Syngelaki A, et al. Comparison of diagnostic accuracy of early screening for pre‐eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: results of SPREE. Ultrasound Obstet Gynecol. 2018;51:743‐750. - PubMed
-
- Serra B, Mendoza M, Scazzocchio E, et al. A new model for screening for early‐onset preeclampsia. Am J Obstet Gynecol. 2020;222:608.e1‐608.e18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

