Intravitreal injection of a rationally designed AAV capsid library in non-human primate identifies variants with enhanced retinal transduction and neutralizing antibody evasion
- PMID: 37814449
- PMCID: PMC10727955
- DOI: 10.1016/j.ymthe.2023.10.001
Intravitreal injection of a rationally designed AAV capsid library in non-human primate identifies variants with enhanced retinal transduction and neutralizing antibody evasion
Abstract
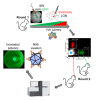
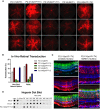
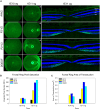
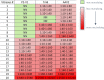
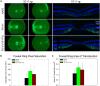
Adeno-associated virus (AAV) continues to be the gold standard vector for therapeutic gene delivery and has proven especially useful for treating ocular disease. Intravitreal injection (IVtI) is a promising delivery route because it increases accessibility of gene therapies to larger patient populations. However, data from clinical and non-human primate (NHP) studies utilizing currently available capsids indicate that anatomical barriers to AAV and pre-existing neutralizing antibodies can restrict gene expression to levels that are "sub-therapeutic" in a substantial proportion of patients. Here, we performed a combination of directed evolution in NHPs of an AAV2-based capsid library with simultaneous mutations across six surface-exposed variable regions and rational design to identify novel capsid variants with improved retinal transduction following IVtI. Following two rounds of screening in NHP, enriched variants were characterized in intravitreally injected mice and NHPs and shown to have increased transduction relative to AAV2. Lead capsid variant, P2-V1, demonstrated an increased ability to evade neutralizing antibodies in human vitreous samples relative to AAV2 and AAV2.7m8. Taken together, this study further contributed to our understanding of the selective pressures associated with retinal transduction via the vitreous and identified promising novel AAV capsid variants for clinical consideration.
Keywords: AAV; adeno-associated virus; capsid library; directed evolution; intravitreal; neutralizing antibody; retina.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests S.E.B. and S.L.B. are cofounders of Atsena Therapeutics, a company that has in-licensed technology described in this report. S.E.B. and S.L.B. are both recipients of research funding from Atsena Therapeutics. S.Z., D.M., P.D.G., S.E.B., and S.L.B. are inventors on a patent (US 10,793,606) associated with this work.
Figures



References
-
- Dalkara D., Byrne L.C., Klimczak R.R., Visel M., Yin L., Merigan W.H., Flannery J.G., Schaffer D.V. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med. 2013;5:189ra76. doi: 10.1126/scitranslmed.3005708. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

