Phenotypic Switching of Vascular Smooth Muscle Cells in Atherosclerosis
- PMID: 37815057
- PMCID: PMC10757534
- DOI: 10.1161/JAHA.123.031121
Phenotypic Switching of Vascular Smooth Muscle Cells in Atherosclerosis
Abstract
The medial layer of the arterial wall is composed mainly of vascular smooth muscle cells (VSMCs). Under physiological conditions, VSMCs assume a contractile phenotype, and their primary function is to regulate vascular tone. In contrast with terminally differentiated cells, VSMCs possess phenotypic plasticity, capable of transitioning into other cellular phenotypes in response to changes in the vascular environment. Recent research has shown that VSMC phenotypic switching participates in the pathogenesis of atherosclerosis, where the various types of dedifferentiated VSMCs accumulate in the atherosclerotic lesion and participate in the associated vascular remodeling by secreting extracellular matrix proteins and proteases. This review article discusses the 9 VSMC phenotypes that have been reported in atherosclerotic lesions and classifies them into differentiated VSMCs, intermediately dedifferentiated VSMCs, and dedifferentiated VSMCs. It also provides an overview of several methodologies that have been developed for studying VSMC phenotypic switching and discusses their respective advantages and limitations.
Keywords: atherosclerosis; cell lineage; dedifferentiation; phenotypes; vascular smooth muscle cells.
Figures
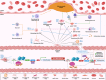
 represents exacerbation, and T represents inhibition of atherosclerosis. The blue font represents the marker of each phenotype. 5hmC indicates 5‐hydroxymethylcytosine; α‐SMA, α‐smooth muscle actin; ABCA1, ATP‐binding cassette transporter 1; Bgn, biglycan; CArG, CC(A/T‐rich)6GG cis‐regulating element; cbfa1, core‐binding factor α1; CD68, cluster of differentiation 68; CNN, calponin; Col1a1, collagen type I alpha 1 chain; Col1a2, collagen type I alpha 2 chain; Dcn, decorin; ECM, extracellular matrix; ELK; ETS transcription factor ELK1; Eng, endoglin; Fn1, fibronectin 1; GC, G/C repressor element; H3K4me2, histone H3 lysine 4 di‐methylation; HO‐1, heme oxygenase 1; KLF4, Krüppel‐like factor 4; Lgals3, galectin 3; Ly6a, lymphocyte antigen 6 family member A; Ly6c1, lymphocyte antigen 6 family member C1; Lum, lumican; Mac3, lysosomal‐associated membrane protein 2; MCP1, monocyte chemoattractant protein 1; Msx2, Msh homeobox 2; Myocd, myocardin; PDGFRβ, platelet‐derived growth factor receptor beta; OPN, osteophosphorin; Runx2, Runt‐related transcription factor 2; S100A4, S100 calcium binding protein A4; Sca‐1, stem cell antigen‐1; SEM‐like VSMC, stem cell/endothelial cell/monocyte cell‐like VSMC;SM22α, transgelin; SM‐MHC, myosin heavy chain 11; SOX9, SRY‐box transcription factor 9; SRF, serum response factor; TCE, transforming growth factor‐beta control elements; TET2, Tet methylcytosine dioxygenase 2; Tsp‐1, thrombospondin‐1; UCP‐1, uncoupling protein 1; Vcam1, vascular cell adhesion molecule 1; and VSMC, vascular smooth muscle cell.
represents exacerbation, and T represents inhibition of atherosclerosis. The blue font represents the marker of each phenotype. 5hmC indicates 5‐hydroxymethylcytosine; α‐SMA, α‐smooth muscle actin; ABCA1, ATP‐binding cassette transporter 1; Bgn, biglycan; CArG, CC(A/T‐rich)6GG cis‐regulating element; cbfa1, core‐binding factor α1; CD68, cluster of differentiation 68; CNN, calponin; Col1a1, collagen type I alpha 1 chain; Col1a2, collagen type I alpha 2 chain; Dcn, decorin; ECM, extracellular matrix; ELK; ETS transcription factor ELK1; Eng, endoglin; Fn1, fibronectin 1; GC, G/C repressor element; H3K4me2, histone H3 lysine 4 di‐methylation; HO‐1, heme oxygenase 1; KLF4, Krüppel‐like factor 4; Lgals3, galectin 3; Ly6a, lymphocyte antigen 6 family member A; Ly6c1, lymphocyte antigen 6 family member C1; Lum, lumican; Mac3, lysosomal‐associated membrane protein 2; MCP1, monocyte chemoattractant protein 1; Msx2, Msh homeobox 2; Myocd, myocardin; PDGFRβ, platelet‐derived growth factor receptor beta; OPN, osteophosphorin; Runx2, Runt‐related transcription factor 2; S100A4, S100 calcium binding protein A4; Sca‐1, stem cell antigen‐1; SEM‐like VSMC, stem cell/endothelial cell/monocyte cell‐like VSMC;SM22α, transgelin; SM‐MHC, myosin heavy chain 11; SOX9, SRY‐box transcription factor 9; SRF, serum response factor; TCE, transforming growth factor‐beta control elements; TET2, Tet methylcytosine dioxygenase 2; Tsp‐1, thrombospondin‐1; UCP‐1, uncoupling protein 1; Vcam1, vascular cell adhesion molecule 1; and VSMC, vascular smooth muscle cell.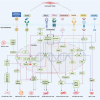
Similar articles
-
Six Shades of Vascular Smooth Muscle Cells Illuminated by KLF4 (Krüppel-Like Factor 4).Arterioscler Thromb Vasc Biol. 2021 Nov;41(11):2693-2707. doi: 10.1161/ATVBAHA.121.316600. Epub 2021 Sep 2. Arterioscler Thromb Vasc Biol. 2021. PMID: 34470477 Free PMC article. Review.
-
Smooth muscle cell-driven vascular diseases and molecular mechanisms of VSMC plasticity.Cell Signal. 2018 Dec;52:48-64. doi: 10.1016/j.cellsig.2018.08.019. Epub 2018 Aug 30. Cell Signal. 2018. PMID: 30172025 Review.
-
Phenotypic switching of vascular smooth muscle cells in atherosclerosis, hypertension, and aortic dissection.J Cell Physiol. 2024 Apr;239(4):e31200. doi: 10.1002/jcp.31200. Epub 2024 Jan 30. J Cell Physiol. 2024. PMID: 38291732 Review.
-
NEAT1 regulates VSMC differentiation and calcification in as long noncoding RNA NEAT1 enhances phenotypic and osteogenic switching of vascular smooth muscle cells in atherosclerosis via scaffolding EZH2.Am J Physiol Cell Physiol. 2024 Jun 1;326(6):C1721-C1734. doi: 10.1152/ajpcell.00587.2023. Epub 2024 Apr 22. Am J Physiol Cell Physiol. 2024. PMID: 38646788 Free PMC article.
-
Vascular Smooth Muscle Cells in Atherosclerosis.Circ Res. 2016 Feb 19;118(4):692-702. doi: 10.1161/CIRCRESAHA.115.306361. Circ Res. 2016. PMID: 26892967 Free PMC article. Review.
Cited by
-
Spatial Transcriptomic Approach to Understanding Coronary Atherosclerotic Plaque Stability.Arterioscler Thromb Vasc Biol. 2024 Nov;44(11):e264-e276. doi: 10.1161/ATVBAHA.123.320330. Epub 2024 Sep 5. Arterioscler Thromb Vasc Biol. 2024. PMID: 39234691
-
Differential aortic aneurysm formation provoked by chemogenetic oxidative stress.J Clin Invest. 2025 Mar 18;135(9):e188743. doi: 10.1172/JCI188743. eCollection 2025 May 1. J Clin Invest. 2025. PMID: 40100302 Free PMC article.
-
JOSD2 inhibits angiotensin II-induced vascular remodeling by deubiquitinating and stabilizing SMAD7.Acta Pharmacol Sin. 2025 May;46(5):1275-1288. doi: 10.1038/s41401-024-01437-y. Epub 2025 Jan 20. Acta Pharmacol Sin. 2025. PMID: 39833306
-
Amino Acid Metabolism and Autophagy in Atherosclerotic Cardiovascular Disease.Biomolecules. 2024 Dec 6;14(12):1557. doi: 10.3390/biom14121557. Biomolecules. 2024. PMID: 39766264 Free PMC article. Review.
-
Regulation of Vascular Injury and Repair by P21-Activated Kinase 1 and P21-Activated Kinase 2: Therapeutic Potential and Challenges.Biomolecules. 2024 Dec 13;14(12):1596. doi: 10.3390/biom14121596. Biomolecules. 2024. PMID: 39766303 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

