A Multilevel Primary Care Intervention to Improve Follow-Up of Overdue Abnormal Cancer Screening Test Results: A Cluster Randomized Clinical Trial
- PMID: 37815566
- PMCID: PMC10565610
- DOI: 10.1001/jama.2023.18755
A Multilevel Primary Care Intervention to Improve Follow-Up of Overdue Abnormal Cancer Screening Test Results: A Cluster Randomized Clinical Trial
Abstract
Importance: Realizing the benefits of cancer screening requires testing of eligible individuals and processes to ensure follow-up of abnormal results.
Objective: To test interventions to improve timely follow-up of overdue abnormal breast, cervical, colorectal, and lung cancer screening results.
Design, setting, and participants: Pragmatic, cluster randomized clinical trial conducted at 44 primary care practices within 3 health networks in the US enrolling patients with at least 1 abnormal cancer screening test result not yet followed up between August 24, 2020, and December 13, 2021.
Intervention: Automated algorithms developed using data from electronic health records (EHRs) recommended follow-up actions and times for abnormal screening results. Primary care practices were randomized in a 1:1:1:1 ratio to (1) usual care, (2) EHR reminders, (3) EHR reminders and outreach (a patient letter was sent at week 2 and a phone call at week 4), or (4) EHR reminders, outreach, and navigation (a patient letter was sent at week 2 and a navigator outreach phone call at week 4). Patients, physicians, and practices were unblinded to treatment assignment.
Main outcomes and measures: The primary outcome was completion of recommended follow-up within 120 days of study enrollment. The secondary outcomes included completion of recommended follow-up within 240 days of enrollment and completion of recommended follow-up within 120 days and 240 days for specific cancer types and levels of risk.
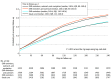
Results: Among 11 980 patients (median age, 60 years [IQR, 52-69 years]; 64.8% were women; 83.3% were White; and 15.4% were insured through Medicaid) with an abnormal cancer screening test result for colorectal cancer (8245 patients [69%]), cervical cancer (2596 patients [22%]), breast cancer (1005 patients [8%]), or lung cancer (134 patients [1%]) and abnormal test results categorized as low risk (6082 patients [51%]), medium risk (3712 patients [31%]), or high risk (2186 patients [18%]), the adjusted proportion who completed recommended follow-up within 120 days was 31.4% in the EHR reminders, outreach, and navigation group (n = 3455), 31.0% in the EHR reminders and outreach group (n = 2569), 22.7% in the EHR reminders group (n = 3254), and 22.9% in the usual care group (n = 2702) (adjusted absolute difference for comparison of EHR reminders, outreach, and navigation group vs usual care, 8.5% [95% CI, 4.8%-12.0%], P < .001). The secondary outcomes showed similar results for completion of recommended follow-up within 240 days and by subgroups for cancer type and level of risk for the abnormal screening result.
Conclusions and relevance: A multilevel primary care intervention that included EHR reminders and patient outreach with or without patient navigation improved timely follow-up of overdue abnormal cancer screening test results for breast, cervical, colorectal, and lung cancer.
Trial registration: ClinicalTrials.gov Identifier: NCT03979495.
Conflict of interest statement
Figures

References
-
- American Cancer Society . American Cancer Society guidelines for the early detection of cancer. Published February 24, 2023. Accessed July 24, 2023. https://www.cancer.org/cancer/screening/american-cancer-society-guidelin...
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

