Nasal Iodophor Antiseptic vs Nasal Mupirocin Antibiotic in the Setting of Chlorhexidine Bathing to Prevent Infections in Adult ICUs: A Randomized Clinical Trial
- PMID: 37815567
- PMCID: PMC10565599
- DOI: 10.1001/jama.2023.17219
Nasal Iodophor Antiseptic vs Nasal Mupirocin Antibiotic in the Setting of Chlorhexidine Bathing to Prevent Infections in Adult ICUs: A Randomized Clinical Trial
Abstract
Importance: Universal nasal mupirocin plus chlorhexidine gluconate (CHG) bathing in intensive care units (ICUs) prevents methicillin-resistant Staphylococcus aureus (MRSA) infections and all-cause bloodstream infections. Antibiotic resistance to mupirocin has raised questions about whether an antiseptic could be advantageous for ICU decolonization.
Objective: To compare the effectiveness of iodophor vs mupirocin for universal ICU nasal decolonization in combination with CHG bathing.
Design, setting, and participants: Two-group noninferiority, pragmatic, cluster-randomized trial conducted in US community hospitals, all of which used mupirocin-CHG for universal decolonization in ICUs at baseline. Adult ICU patients in 137 randomized hospitals during baseline (May 1, 2015-April 30, 2017) and intervention (November 1, 2017-April 30, 2019) were included.
Intervention: Universal decolonization involving switching to iodophor-CHG (intervention) or continuing mupirocin-CHG (baseline).
Main outcomes and measures: ICU-attributable S aureus clinical cultures (primary outcome), MRSA clinical cultures, and all-cause bloodstream infections were evaluated using proportional hazard models to assess differences from baseline to intervention periods between the strategies. Results were also compared with a 2009-2011 trial of mupirocin-CHG vs no decolonization in the same hospital network. The prespecified noninferiority margin for the primary outcome was 10%.
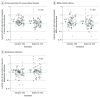
Results: Among the 801 668 admissions in 233 ICUs, the participants' mean (SD) age was 63.4 (17.2) years, 46.3% were female, and the mean (SD) ICU length of stay was 4.8 (4.7) days. Hazard ratios (HRs) for S aureus clinical isolates in the intervention vs baseline periods were 1.17 for iodophor-CHG (raw rate: 5.0 vs 4.3/1000 ICU-attributable days) and 0.99 for mupirocin-CHG (raw rate: 4.1 vs 4.0/1000 ICU-attributable days) (HR difference in differences significantly lower by 18.4% [95% CI, 10.7%-26.6%] for mupirocin-CHG, P < .001). For MRSA clinical cultures, HRs were 1.13 for iodophor-CHG (raw rate: 2.3 vs 2.1/1000 ICU-attributable days) and 0.99 for mupirocin-CHG (raw rate: 2.0 vs 2.0/1000 ICU-attributable days) (HR difference in differences significantly lower by 14.1% [95% CI, 3.7%-25.5%] for mupirocin-CHG, P = .007). For all-pathogen bloodstream infections, HRs were 1.00 (2.7 vs 2.7/1000) for iodophor-CHG and 1.01 (2.6 vs 2.6/1000) for mupirocin-CHG (nonsignificant HR difference in differences, -0.9% [95% CI, -9.0% to 8.0%]; P = .84). Compared with the 2009-2011 trial, the 30-day relative reduction in hazards in the mupirocin-CHG group relative to no decolonization (2009-2011 trial) were as follows: S aureus clinical cultures (current trial: 48.1% [95% CI, 35.6%-60.1%]; 2009-2011 trial: 58.8% [95% CI, 47.5%-70.7%]) and bloodstream infection rates (current trial: 70.4% [95% CI, 62.9%-77.8%]; 2009-2011 trial: 60.1% [95% CI, 49.1%-70.7%]).
Conclusions and relevance: Nasal iodophor antiseptic did not meet criteria to be considered noninferior to nasal mupirocin antibiotic for the outcome of S aureus clinical cultures in adult ICU patients in the context of daily CHG bathing. In addition, the results were consistent with nasal iodophor being inferior to nasal mupirocin.
Trial registration: ClinicalTrials.gov Identifier: NCT03140423.
Conflict of interest statement
Figures

References
-
- Weiner-Lastinger LM, Abner S, Edwards JR, et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: summary of data reported to the National Healthcare Safety Network, 2015-2017. Infect Control Hosp Epidemiol. 2020;41(1):1-18. doi: 10.1017/ice.2019.296 - DOI - PMC - PubMed
-
- Kourtis AP, Hatfield K, Baggs J, et al. ; Emerging Infections Program MRSA author group . Vital signs: epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections: United States. MMWR Morb Mortal Wkly Rep. 2019;68(9):214-219. doi: 10.15585/mmwr.mm6809e1 - DOI - PMC - PubMed
-
- Russell D, Beekmann SE, Polgreen PM, Rubin Z, Uslan DZ. Routine use of contact precautions for methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: which way is the pendulum swinging? Infect Control Hosp Epidemiol. 2016;37(1):36-40. doi: 10.1017/ice.2015.246 - DOI - PubMed

