Desensitization and remission after peanut sublingual immunotherapy in 1- to 4-year-old peanut-allergic children: A randomized, placebo-controlled trial
- PMID: 37815782
- PMCID: PMC10872748
- DOI: 10.1016/j.jaci.2023.08.032
Desensitization and remission after peanut sublingual immunotherapy in 1- to 4-year-old peanut-allergic children: A randomized, placebo-controlled trial
Abstract
Background: Prior studies of peanut sublingual immunotherapy (SLIT) have suggested a potential advantage with younger age at treatment initiation.
Objective: We studied the safety and efficacy of SLIT for peanut allergy in 1- to 4-year-old children.
Methods: Peanut-allergic 1- to 4-year-old children were randomized to receive 4 mg peanut SLIT versus placebo. Desensitization was assessed by double-blind, placebo-controlled food challenge (DBPCFC) after 36 months of treatment. Participants desensitized to at least 443 mg peanut protein discontinued therapy for 3 months and then underwent DBPCFC to assess for remission. Biomarkers were measured at baseline and longitudinally during treatment.
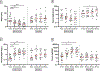
Results: Fifty participants (25 peanut SLIT, 25 placebo) with a median age of 2.4 years were enrolled across 2 sites. The primary end point of desensitization was met with actively treated versus placebo participants having a significantly greater median cumulative tolerated dose (4443 mg vs 143 mg), higher likelihood of passing the month 36 DBPCFC (60% vs 0), and higher likelihood of demonstrating remission (48% vs 0). The highest rate of desensitization and remission was seen in 1- to 2-year-olds, followed by 2- to 3-year-olds and 3- to 4-year-olds. Longitudinal changes in peanut skin prick testing, peanut-specific IgG4, and peanut-specific IgG4/IgE ratio were seen in peanut SLIT but not placebo participants. Oropharyngeal itching was more commonly reported by peanut SLIT than placebo participants. Skin, gastrointestinal, upper respiratory, lower respiratory, and multisystem adverse events were similar between treatment groups.
Conclusion: Peanut SLIT safely induces desensitization and remission in 1- to 4-year-old children, with improved outcomes seen with younger age at initiation.
Keywords: Peanut allergy; SLIT; desensitization; food allergy; food immunotherapy; remission; sublingual immunotherapy.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
CONFLICTS OF INTEREST
EHK reports advisory board membership with ALK-Abello, Kenota Health, and Ukko Inc; consultancy with AllerGenis, Allergy Therapeutics Ltd, Belhaven BioPharma, Duke Clinical Research Institute, Genentech, Nutricia, and Revolo; and receives grant funding to his institution from the National Center for Complementary and Integrative Health (NCCIH), National Institute of Allergy and Infectious Diseases (NIAID), and Food Allergy Research and Education (FARE). JAB reports consultancy with AllerGenis, Allergy Therapeutics Ltd, Before Brands, DBV Technologies, FARE, Genentech, HAL Allergy, Novartis, and Nutricia; and receives grant funding to his institution from Aimmune Therapeutics, Astellas, DBV Technologies, FARE, Genentech, NIAID, Novartis, Regeneron, and Siolta; CAK is an Associate Editor at the Journal of Allergy and Clinical Immunology; is on the Board of the American Board of Allergy and Immunology; and receives royalties from Up-to-Date. MDK reports consultancy with Ukko, Inc. and receives research support to his institution from NIAID and the US Department of Defense. AWB reports advisory board membership with Aimmune Therapeutics, Consortia TX, and Ukko Inc; consultancy with Allergy Therapeutics Ltd and DBV Technologies; royalties from UpToDate, Elsevier, and Wolter Kluwer; and receives grant funding to his institution from NCCIH, NIAID, FARE, and the Burroughs Wellcome Fund. The remaining authors declare no conflicts of interest.
Figures



Comment in
-
The promise of sublingual and other immunotherapy options for infants and toddlers with food allergy.J Allergy Clin Immunol. 2024 Jan;153(1):95-97. doi: 10.1016/j.jaci.2023.11.009. Epub 2023 Nov 20. J Allergy Clin Immunol. 2024. PMID: 37992817 No abstract available.
References
-
- Neuman-Sunshine DL, Eckman JA, Keet CA, Matsui EC, Peng RD, Lenehan PJ, et al. The natural history of persistent peanut allergy. Ann Allergy Asthma Immunol. 2012;108(5):326–31 e3. - PubMed
-
- Gupta R, Holdford D, Bilaver L, Dyer A, Holl JL, Meltzer D. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167(11):1026–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

