Intraventricular Lavage vs External Ventricular Drainage for Intraventricular Hemorrhage: A Randomized Clinical Trial
- PMID: 37815832
- PMCID: PMC10565600
- DOI: 10.1001/jamanetworkopen.2023.35247
Intraventricular Lavage vs External Ventricular Drainage for Intraventricular Hemorrhage: A Randomized Clinical Trial
Abstract
Importance: Intraventricular lavage has been proposed as a minimally invasive method to evacuate intraventricular hemorrhage. There is little evidence to support its use.
Objective: To evaluate the safety and potential efficacy of intraventricular lavage treatment of intraventricular hemorrhage.
Design, setting, and participants: This single-blinded, controlled, investigator-initiated 1:1 randomized clinical trial was conducted at Aarhus University Hospital and Odense University Hospital in Denmark from January 13, 2022, to November 24, 2022. Follow-up duration was 90 days. The trial was set to include 58 patients with intraventricular hemorrhage. Prespecified interim analysis was performed for the first 20 participants. Data were analyzed from February to April 2023.
Interventions: Participants were randomized to receive either intraventricular lavage or standard drainage.
Main outcomes and measures: The main outcome was risk of catheter occlusions. Additional safety outcomes were catheter-related infections and procedure time, length of stay at the intensive care unit, duration of treatment, and 30-day mortality. The main outcome of the prespecified interim analysis was risk of severe adverse events. Efficacy outcomes were hematoma clearance, functional outcome, overall survival, and shunt dependency.
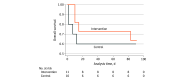
Results: A total of 21 participants (median [IQR] age, 67 [59-82] years; 14 [66%] male) were enrolled, with 11 participants randomized to intraventricular lavage and 10 participants randomized to standard drainage; 20 participants (95%) had secondary intraventricular hemorrhage. The median (IQR) Graeb score was 9 (5-11), and the median (IQR) Glasgow Coma Scale score was 6.5 (4-8). The study was terminated early due to a significantly increased risk of severe adverse events associated with intraventricular lavage at interim analysis (risk difference for control vs intervention, 0.43; 95% CI, 0.06-0.81; P = .04; incidence rate ratio for control vs intervention, 6.0; 95% CI, 1.38-26.1; P = .01). The rate of catheter occlusion was higher for intraventricular lavage compared with drainage (6 of 16 patients [38%] vs 2 of 13 patients [7%]; hazard ratio, 4.4 [95% CI, 0.6-31.2]; P = .14), which met the prespecified α = .20 level. Median (IQR) procedure time for catheter placement was 53.5 (33-75) minutes for intraventricular lavage vs 12 (4-20) minutes for control (P < .001).
Conclusions and relevance: This randomized clinical trial of intraventricular lavage vs standard drainage found that intraventricular lavage was encumbered with a significantly increased number of severe adverse events. Caution is recommended when using the device to ensure patient safety.
Trial registration: ClinicalTrials.gov Identifier: NCT05204849.
Conflict of interest statement
Figures
Comment in
-
Computer-Supervised EVD Raises Safety Questions in ICU Care of IVH: Humans-1, Computers-0.JAMA Netw Open. 2023 Oct 2;6(10):e2335184. doi: 10.1001/jamanetworkopen.2023.35184. JAMA Netw Open. 2023. PMID: 37815834 No abstract available.
References
-
- Yamaguchi Y, Nakagawa K, Watanabe H, et al. . Primary intraventricular hemorrhage. Article in Japanese. Jpn J Neurosurg. 2000;9(10):672-678. doi:10.7887/jcns.9.672 - DOI
-
- Haldrup M, Miscov R, Mohamad N, et al. . Treatment of intraventricular hemorrhage with external ventricular drainage and fibrinolysis: a comprehensive systematic review and meta-analysis of complications and outcome. World Neurosurg. 2023;174:183-196.e6. doi:10.1016/j.wneu.2023.01.021 - DOI - PubMed
-
- Hanley DF, Lane K, McBee N, et al. ; CLEAR III Investigators . Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet. 2017;389(10069):603-611. doi:10.1016/S0140-6736(16)32410-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous