GADD45A is a mediator of mitochondrial loss, atrophy, and weakness in skeletal muscle
- PMID: 37815864
- PMCID: PMC10721312
- DOI: 10.1172/jci.insight.171772
GADD45A is a mediator of mitochondrial loss, atrophy, and weakness in skeletal muscle
Abstract
Aging and many illnesses and injuries impair skeletal muscle mass and function, but the molecular mechanisms are not well understood. To better understand the mechanisms, we generated and studied transgenic mice with skeletal muscle-specific expression of growth arrest and DNA damage inducible α (GADD45A), a signaling protein whose expression in skeletal muscle rises during aging and a wide range of illnesses and injuries. We found that GADD45A induced several cellular changes that are characteristic of skeletal muscle atrophy, including a reduction in skeletal muscle mitochondria and oxidative capacity, selective atrophy of glycolytic muscle fibers, and paradoxical expression of oxidative myosin heavy chains despite mitochondrial loss. These cellular changes were at least partly mediated by MAP kinase kinase kinase 4, a protein kinase that is directly activated by GADD45A. By inducing these changes, GADD45A decreased the mass of muscles that are enriched in glycolytic fibers, and it impaired strength, specific force, and endurance exercise capacity. Furthermore, as predicted by data from mouse models, we found that GADD45A expression in skeletal muscle was associated with muscle weakness in humans. Collectively, these findings identify GADD45A as a mediator of mitochondrial loss, atrophy, and weakness in mouse skeletal muscle and a potential target for muscle weakness in humans.
Keywords: Metabolism; Mitochondria; Muscle Biology; Skeletal muscle.
Conflict of interest statement
Figures

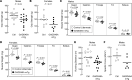


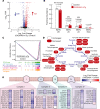
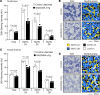
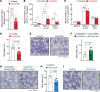


References
Publication types
MeSH terms
Substances
Grants and funding
- I01 BX000976/BX/BLRD VA/United States
- R01 DK119167/DK/NIDDK NIH HHS/United States
- R01 AG064386/AG/NIA NIH HHS/United States
- R01 DK133194/DK/NIDDK NIH HHS/United States
- R01 AG054454/AG/NIA NIH HHS/United States
- R13 AG071150/AG/NIA NIH HHS/United States
- R01 DK133401/DK/NIDDK NIH HHS/United States
- R01 AR071762/AR/NIAMS NIH HHS/United States
- R01 AG060637/AG/NIA NIH HHS/United States
- P30 AG013319/AG/NIA NIH HHS/United States
- I01 RX001477/RX/RRD VA/United States
- UL1 TR001439/TR/NCATS NIH HHS/United States
- R44 AG047684/AG/NIA NIH HHS/United States
- R44 AR069400/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

