Plasma IgG aggregates as biomarkers for multiple sclerosis
- PMID: 37816415
- PMCID: PMC11830478
- DOI: 10.1016/j.clim.2023.109801
Plasma IgG aggregates as biomarkers for multiple sclerosis
Abstract
We recently reported that multiple sclerosis (MS) plasma contains IgG aggregates and induces complement-dependent neuronal cytotoxicity (Zhou et al., 2023). Using ELISA, we report herein that plasma IgG levels in the aggregates can be used as biomarkers for MS. We enriched the IgG aggregates from samples of two cohorts (190 MS and 160 controls) by collecting flow-through after plasma binding to Protein A followed by detection of IgG subclass. We show that there are significantly higher levels of IgG1, IgG3, and total IgG antibodies in MS IgG aggregates, with an AUC >90%; higher levels of IgG1 distinguish secondary progressive MS from relapsing-remitting MS (AUC = 91%). Significantly, we provided the biological rationale for MS plasma IgG biomarkers by demonstrating the strong correlation between IgG antibodies and IgG aggregate-induced neuronal cytotoxicity. These non-invasive, simple IgG-based blood ELISA assays can be adapted into clinical practice for diagnosing MS and SPMS and monitoring treatment responses.
Keywords: Antibody; Biomarker; Cytotoxicity; Diagnosis; IgG; IgG aggregates; IgG1; Immunoglobulin; Multiple sclerosis; Plasma; Progressive MS; SPMS.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest We declare that there are no conflicts of interest for all authors.
Figures
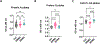
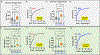

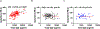


References
-
- Link H, Huang YM, Oligoclonal bands in multiple sclerosis cerebrospinal fluid: an update on methodology and clinical usefulness, J. Neuroimmunol 180 (2006) 17–28. - PubMed
-
- Farina G, Magliozzi R, Pitteri M, Reynolds R, Rossi S, Gajofatto A, Benedetti MD, Facchiano F, Monaco S, Calabrese M, Increased cortical lesion load and intrathecal inflammation is associated with oligoclonal bands in multiple sclerosis patients: a combined CSF and MRI study, J. Neuroinflammation 14 (2017) 40. - PMC - PubMed
-
- Ferreira D, Voevodskaya O, Imrell K, Stawiarz L, Spulber G, Wahlund LO, Hillert J, Westman E, Karrenbauer VD, Multiple sclerosis patients lacking oligoclonal bands in the cerebrospinal fluid have less global and regional brain atrophy, J. Neuroimmunol 274 (2014) 149–154. - PubMed
-
- Caroscio JT, Kochwa S, Sacks H, Makuku S, Cohen JA, Yahr MD, Quantitative cerebrospinal fluid IgG measurements as a marker of disease activity in multiple sclerosis, Arch. Neurol 43 (1986) 1129–1131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

