Inhibition of mitochondrial fission activates glycogen synthesis to support cell survival in colon cancer
- PMID: 37816729
- PMCID: PMC10564897
- DOI: 10.1038/s41419-023-06202-3
Inhibition of mitochondrial fission activates glycogen synthesis to support cell survival in colon cancer
Abstract
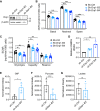
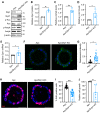
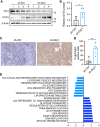
Metabolic reprogramming has been recognized as one of the major mechanisms that fuel tumor initiation and progression. Our previous studies demonstrate that activation of Drp1 promotes fatty acid oxidation and downstream Wnt signaling. Here we investigate the role of Drp1 in regulating glycogen metabolism in colon cancer. Knockdown of Drp1 decreases mitochondrial respiration without increasing glycolysis. Analysis of cellular metabolites reveals that the levels of glucose-6-phosphate, a precursor for glycogenesis, are significantly elevated whereas pyruvate and other TCA cycle metabolites remain unchanged in Drp1 knockdown cells. Additionally, silencing Drp1 activates AMPK to stimulate the expression glycogen synthase 1 (GYS1) mRNA and promote glycogen storage. Using 3D organoids from Apcf/f/Villin-CreERT2 models, we show that glycogen levels are elevated in tumor organoids upon genetic deletion of Drp1. Similarly, increased GYS1 expression and glycogen accumulation are detected in xenograft tumors derived from Drp1 knockdown colon cancer cells. Functionally, increased glycogen storage provides survival advantage to Drp1 knockdown cells. Co-targeting glycogen phosphorylase-mediated glycogenolysis sensitizes Drp1 knockdown cells to chemotherapy drug treatment. Taken together, our results suggest that Drp1-loss activates glucose uptake and glycogenesis as compensative metabolic pathways to promote cell survival. Combined inhibition of glycogen metabolism may enhance the efficacy of chemotherapeutic agents for colon cancer treatment.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

