Macrophages originated IL-33/ST2 inhibits ferroptosis in endometriosis via the ATF3/SLC7A11 axis
- PMID: 37816731
- PMCID: PMC10564909
- DOI: 10.1038/s41419-023-06182-4
Macrophages originated IL-33/ST2 inhibits ferroptosis in endometriosis via the ATF3/SLC7A11 axis
Erratum in
-
Correction: Macrophages originated IL-33/ST2 inhibits ferroptosis in endometriosis via the ATF3/SLC7A11 axis.Cell Death Dis. 2025 Mar 27;16(1):212. doi: 10.1038/s41419-025-07484-5. Cell Death Dis. 2025. PMID: 40148318 Free PMC article. No abstract available.
Abstract
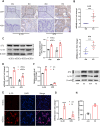
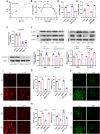
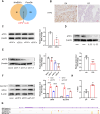
Endometriosis is a gynecological inflammatory disease that is linked with immune cells, specifically macrophages. IL-33 secreted from macrophages is known to accelerate the progression of endometriosis. The periodic and repeated bleeding that occurs in women with endometriosis leads to excess iron in the microenvironment that is conducive to ferroptosis, a process related to intracellular ROS production, lipid peroxidation and mitochondrial damage. It is suggested that eESCs may specifically be able to inhibit ferroptosis. However, it is currently unclear whether IL-33 directly regulates ferroptosis to influence the disease course in endometriosis. In this study, eESCs co-cultured with macrophages or stimulated with IL-33/ST2 were observed to have increased cell viability and migration. Additionally, IL-33/ST2 decreased intracellular iron levels and lipid peroxidation in eESCs exposed to erastin treatment. Furthermore, IL-33/ST2 treatment resulted in a notable upregulation in SLC7A11 expression in eESCs due to the downregulation of negative transcription factor ATF3, thereby suppressing ferroptosis. The P38/JNK pathway activated by IL-33/ST2 was also found to inhibit the transcription factor ATF3. Therefore, we concluded that IL-33/ST2 inhibits the ATF3-mediated reduction in SLC7A11 transcript levels via the P38/JNK pathway. The findings reveal that macrophage-derived IL-33 upregulates SLC7A11 in eESCs through the p38/JNK/ATF3 pathway, ultimately resulting in protection against ferroptosis in eESCs. Moreover, we conducted an experiment using endometriosis model mice that showed that a combination of IL-33-Ab and erastin treatment alleviated the disease, showing the promise of combining immunotherapy and ferroptosis therapy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382:1244–56. - PubMed
-
- Ye L, Whitaker LHR, Mawson RL, Hickey M. Endometriosis. BMJ. 2022;379:e068950. - PubMed
-
- Yoshino O, Izumi G, Shi J, Osuga Y, Hirota Y, Hirata T, et al. Activin-A is induced by interleukin-1β and tumor necrosis factor-α and enhances the mRNA expression of interleukin-6 and protease-activated receptor-2 and proliferation of stromal cells from endometrioma. Fertil Steril. 2011;96:118–21. - PubMed
-
- Bersinger NA, Günthert AR, McKinnon B, Johann S, Mueller MD. Dose-response effect of interleukin (IL)-1β, tumour necrosis factor (TNF)-α, and interferon-γ on the in vitro production of epithelial neutrophil activating peptide-78 (ENA-78), IL-8, and IL-6 by human endometrial stromal cells. Arch Gynecol Obstet. 2011;283:1291–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

