Aldolase B-driven lactagenesis and CEACAM6 activation promote cell renewal and chemoresistance in colorectal cancer through the Warburg effect
- PMID: 37816733
- PMCID: PMC10564793
- DOI: 10.1038/s41419-023-06187-z
Aldolase B-driven lactagenesis and CEACAM6 activation promote cell renewal and chemoresistance in colorectal cancer through the Warburg effect
Abstract
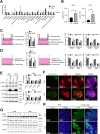
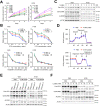
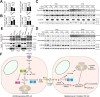
Colorectal cancer (CRC) is a prevalent malignancy worldwide and is associated with a high mortality rate. Changes in bioenergy metabolism, such as the Warburg effect, are often observed in CRC. Aldolase B (ALDOB) has been identified as a potential regulator of these changes, but its exact role in CRC cell behavior and bioenergetic homeostasis is not fully understood. To investigate this, two cohorts of CRC patients were analyzed independently. The results showed that higher ALDOB expression was linked to unfavorable prognosis, increased circulating carcinoembryonic antigen (CEA) levels, and altered bioenergetics in CRC. Further analysis using cell-based assays demonstrated that ALDOB promoted cell proliferation, chemoresistance, and increased expression of CEA in CRC cells. The activation of pyruvate dehydrogenase kinase-1 (PDK1) by ALDOB-induced lactagenesis and secretion, which in turn mediated the effects on CEA expression. Secreted lactate was found to enhance lactate dehydrogenase B (LDHB) expression in adjacent cells and to be a crucial modulator of ALDOB-mediated phenotypes. Additionally, the effect of ALDOB on CEA expression was downstream of the bioenergetic changes mediated by secreted lactate. The study also identified CEA cell adhesion molecule-6 (CEACAM6) as a downstream effector of ALDOB that controlled CRC cell proliferation and chemoresistance. Notably, CEACAM6 activation was shown to enhance protein stability through lysine lactylation, downstream of ALDOB-mediated lactagenesis. The ALDOB/PDK1/lactate/CEACAM6 axis plays an essential role in CRC cell behavior and bioenergetic homeostasis, providing new insights into the involvement of CEACAM6 in CRC and the Warburg effect. These findings may lead to the development of new treatment strategies for CRC patients.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

