Calbindin 2-specific deletion of arginase 2 preserves visual function after optic nerve crush
- PMID: 37816735
- PMCID: PMC10564748
- DOI: 10.1038/s41419-023-06180-6
Calbindin 2-specific deletion of arginase 2 preserves visual function after optic nerve crush
Abstract
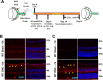
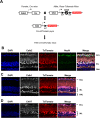
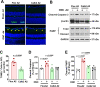
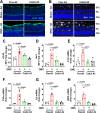
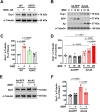
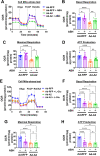
We previously found that global deletion of the mitochondrial enzyme arginase 2 (A2) limits optic nerve crush (ONC)-induced neuronal death. Herein, we examined the cell-specific role of A2 in this pathology by studies using wild type (WT), neuronal-specific calbindin 2 A2 KO (Calb2cre/+ A2 f/f), myeloid-specific A2 KO (LysMcre/+ A2f/f), endothelial-specific A2 KO (Cdh5cre/+ A2f/f), and floxed controls. We also examined the impact of A2 overexpression on mitochondrial function in retinal neuronal R28 cells. Immunolabeling showed increased A2 expression in ganglion cell layer (GCL) neurons of WT mice within 6 h-post injury and inner retinal neurons after 7 days. Calb2 A2 KO mice showed improved neuronal survival, decreased TUNEL-positive neurons, and improved retinal function compared to floxed littermates. Neuronal loss was unchanged by A2 deletion in myeloid or endothelial cells. We also found increased expression of neurotrophins (BDNF, FGF2) and improved survival signaling (pAKT, pERK1/2) in Calb2 A2 KO retinas within 24-hour post-ONC along with suppression of inflammatory mediators (IL1β, TNFα, IL6, and iNOS) and apoptotic markers (cleavage of caspase3 and PARP). ONC increased GFAP and Iba1 immunostaining in floxed controls, and Calb2 A2 KO dampened this effect. Overexpression of A2 in R28 cells increased Drp1 expression, and decreased mitochondrial respiration, whereas ABH-induced inhibition of A2 decreased Drp1 expression and improved mitochondrial respiration. Finally, A2 overexpression or excitotoxic treatment with glutamate significantly impaired mitochondrial function in R28 cells as shown by significant reductions in basal respiration, maximal respiration, and ATP production. Further, glutamate treatment of A2 overexpressing cells did not induce further deterioration in their mitochondrial function, indicating that A2 overexpression or glutamate insult induce comparable alterations in mitochondrial function. Our data indicate that neuronal A2 expression is neurotoxic after injury, and A2 deletion in Calb2 expressing neurons limits ONC-induced retinal neurodegeneration and improves visual function.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

