Knockout of the C3a receptor protects against renal ischemia reperfusion injury by reduction of NETs formation
- PMID: 37816851
- PMCID: PMC11072185
- DOI: 10.1007/s00018-023-04967-6
Knockout of the C3a receptor protects against renal ischemia reperfusion injury by reduction of NETs formation
Abstract
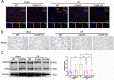
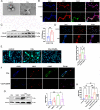
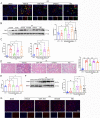
Renal ischemia/reperfusion (I/R) injury is a local sterile inflammatory response driven by innate immunity. Emerging data have revealed that complement and neutrophils contribute to hyperinflammation and oxidative stress in I/R induced acute kidney injury (AKI). However, the interplay between the C3a/C3aR axis and neutrophil extracellular traps (NETs) is imcompletelyunderstood. Here, we utilize genetically engineered mouse models and pharmacological inhibitors to investigate this association. The C3a/C3aR axis is found to promote neutrophil recruitment and NETs formation, thereby accelerating renal damage and dysfunction. Knockout of C3aR restores NETs release and improves renal function after I/R injury. Antibody-mediated blockade of NETs can also significantly ameliorate renal tubular injury and inflammation. Consistently, under stimulation by C3a, neutrophils are activated to promote NETs formation and subsequent renal tubular epithelial cell damage, and blocking C3aR rescued the injury. Interfering with reactive oxygen species (ROS) accumulation in neutrophils by antioxidant treatment significantly attenuates NETs formation. Our findings demonstrate that the C3a/C3aR-ROS-NETs axis constitutes a promising target for prevention or treatment of renal I/R injury.
Keywords: Acute kidney injury; Complement component 3a receptor; Ischemia–reperfusion injury; Neutrophil extracellular traps; Reactive oxygen species.
© 2023. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declared no conflict of interest associated with this study.
Figures







References
-
- Casiraghi F, Azzollini N, Todeschini M, Fiori S, Cavinato RA, Cassis P, Solini S, Pezzuto F, Mister M, Thurman JM, Benigni A, Remuzzi G, Noris M. Complement alternative pathway deficiency in recipients protects kidney allograft from ischemia/reperfusion injury and alloreactive T cell response. Am J Transplant. 2017;17:2312–2325. doi: 10.1111/ajt.14262. - DOI - PubMed
-
- Nakazawa D, Kumar SV, Marschner J, Desai J, Holderied A, Rath L, Kraft F, Lei Y, Fukasawa Y, Moeckel GW, Angelotti ML, Liapis H, Anders HJ. Histones and neutrophil extracellular traps enhance tubular necrosis and remote organ injury in ischemic AKI. J Am Soc Nephrol. 2017;28:1753–1768. doi: 10.1681/ASN.2016080925. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

