CD13 facilitates immune cell migration and aggravates acute injury but promotes chronic post-stroke recovery
- PMID: 37817190
- PMCID: PMC10566099
- DOI: 10.1186/s12974-023-02918-3
CD13 facilitates immune cell migration and aggravates acute injury but promotes chronic post-stroke recovery
Abstract
Introduction: Acute stroke leads to the activation of myeloid cells. These cells express adhesion molecules and transmigrate to the brain, thereby aggravating injury. Chronically after stroke, repair processes, including angiogenesis, are activated and enhance post-stroke recovery. Activated myeloid cells express CD13, which facilitates their migration into the site of injury. However, angiogenic blood vessels which play a role in recovery also express CD13. Overall, the specific contribution of CD13 to acute and chronic stroke outcomes is unknown.
Methods: CD13 expression was estimated in both mice and humans after the ischemic stroke. Young (8-12 weeks) male wild-type and global CD13 knockout (KO) mice were used for this study. Mice underwent 60 min of middle cerebral artery occlusion (MCAO) followed by reperfusion. For acute studies, the mice were euthanized at either 24- or 72 h post-stroke. For chronic studies, the Y-maze, Barnes maze, and the open field were performed on day 7 and day 28 post-stroke. Mice were euthanized at day 30 post-stroke and the brains were collected for assessment of inflammation, white matter injury, tissue loss, and angiogenesis. Flow cytometry was performed on days 3 and 7 post-stroke to quantify infiltrated monocytes and neutrophils and CXCL12/CXCR4 signaling.
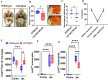
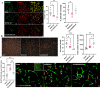
Results: Brain CD13 expression and infiltrated CD13+ monocytes and neutrophils increased acutely after the stroke. The brain CD13+lectin+ blood vessels increased on day 15 after the stroke. Similarly, an increase in the percentage area CD13 was observed in human stroke patients at the subacute time after stroke. Deletion of CD13 resulted in reduced infarct volume and improved neurological recovery after acute stroke. However, CD13KO mice had significantly worse memory deficits, amplified gliosis, and white matter damage compared to wild-type animals at chronic time points. CD13-deficient mice had an increased percentage of CXCL12+cells but a reduced percentage of CXCR4+cells and decreased angiogenesis at day 30 post-stroke.
Conclusions: CD13 is involved in the trans-migration of monocytes and neutrophils after stroke, and acutely, led to decreased infarct size and improved behavioral outcomes. However, loss of CD13 led to reductions in post-stroke angiogenesis by reducing CXCL12/CXCR4 signaling.
Keywords: Angiogenesis; CD13; CXCL12; CXCR4; Cognitive impairment; Ischemic stroke; Myeloid cells; Stroke recovery; Transmigration.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

