Glucose upregulates amphiregulin in oral dysplastic keratinocytes: A potential role in diabetes-associated oral carcinogenesis
- PMID: 37817274
- PMCID: PMC10841538
- DOI: 10.1111/jop.13493
Glucose upregulates amphiregulin in oral dysplastic keratinocytes: A potential role in diabetes-associated oral carcinogenesis
Abstract
Background: Compelling evidence implicates diabetes-associated hyperglycemia as a promoter of tumor progression in oral potentially malignant disorders (OPMD). Yet, information on hyperglycemia-induced cell signaling networks in oral oncology remains limited. Our group recently reported that glucose-rich conditions significantly enhance oral dysplastic keratinocyte viability and migration through epidermal growth factor receptor (EGFR) activation, a pathway strongly linked to oral carcinogenesis. Here, we investigated the basal metabolic phenotype in these cells and whether specific glucose-responsive EGFR ligands mediate these responses.
Methods: Cell energy phenotype and lactate concentration were evaluated via commercially available assays. EGFR ligands in response to normal (5 mM) or high (20 mM) glucose were analyzed by quantitative real-time PCR, ELISA, and western blotting. Cell viability and migration assays were performed in the presence of pharmacological inhibitors or RNA interference.
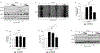
Results: When compared to normal keratinocytes, basal glycolysis in oral dysplastic keratinocytes was significantly elevated. In highly glycolytic cells, high glucose-activated EGFR increasing viability and migration. Notably, we identified amphiregulin (AREG) as the predominant glucose-induced EGFR ligand. Indeed, enhanced cell migration in response to high glucose was blunted by EGFR inhibitor cetuximab and AREG siRNA. Conversely, AREG treatment under normal glucose conditions significantly increased cell viability, migration, lactate levels, and expression of glycolytic marker pyruvate kinase M2.
Conclusion: These novel findings point to AREG as a potential high glucose-induced EGFR activating ligand in highly glycolytic oral dysplastic keratinocytes. Future studies are warranted to gain more insight into the role of AREG in hyperglycemia-associated OPMD tumor progression.
Keywords: EGFR; Oral potentially malignant disorders; amphiregulin; hyperglycemia; oral carcinogenesis.
© 2023 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Figures

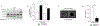
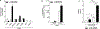


Similar articles
-
Fatty acid synthase mediates high glucose-induced EGFR activation in oral dysplastic keratinocytes.J Oral Pathol Med. 2021 Oct;50(9):919-926. doi: 10.1111/jop.13235. Epub 2021 Aug 23. J Oral Pathol Med. 2021. PMID: 34402100 Free PMC article.
-
Cross-suppression of EGFR ligands amphiregulin and epiregulin and de-repression of FGFR3 signalling contribute to cetuximab resistance in wild-type KRAS tumour cells.Br J Cancer. 2012 Apr 10;106(8):1406-14. doi: 10.1038/bjc.2012.103. Br J Cancer. 2012. PMID: 22491422 Free PMC article.
-
Metalloproteinase-mediated, context-dependent function of amphiregulin and HB-EGF in human keratinocytes and skin.J Invest Dermatol. 2010 Jan;130(1):295-304. doi: 10.1038/jid.2009.211. J Invest Dermatol. 2010. PMID: 19609315 Free PMC article.
-
Amphiregulin.Semin Cell Dev Biol. 2014 Apr;28:31-41. doi: 10.1016/j.semcdb.2014.01.005. Epub 2014 Jan 23. Semin Cell Dev Biol. 2014. PMID: 24463227 Review.
-
Amphiregulin in cellular physiology, health, and disease: Potential use as a biomarker and therapeutic target.J Cell Physiol. 2022 Feb;237(2):1143-1156. doi: 10.1002/jcp.30615. Epub 2021 Oct 26. J Cell Physiol. 2022. PMID: 34698381 Review.
Cited by
-
A review on diabetes and oral cancer: Molecular links and implications.Mol Biol Res Commun. 2025;14(2):109-113. doi: 10.22099/mbrc.2024.51225.2042. Mol Biol Res Commun. 2025. PMID: 40028480 Free PMC article. Review.
-
Basaloid Squamous Cell Carcinoma of the Dorsum of the Tongue Following Chronic Hypertrophic Candidiasis: A Case Report and Literature Review.Cureus. 2025 Apr 24;17(4):e82951. doi: 10.7759/cureus.82951. eCollection 2025 Apr. Cureus. 2025. PMID: 40416125 Free PMC article.
References
-
- Wong T, Wiesenfeld D. Oral Cancer. Aust Dent J. 2018;63 Suppl 1:S91–S99. - PubMed
-
- D’Souza S, Addepalli V. Preventive measures in oral cancer: An overview. Biomed Pharmacother. 2018;107:72–80. - PubMed
-
- Madhura MG, Rao RS, Patil S, Fageeh HN, Alhazmi A, Awan KH. Advanced diagnostic aids for oral cancer. Dis Mon. 2020;66(12):101034. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

