Vascular organoids: unveiling advantages, applications, challenges, and disease modelling strategies
- PMID: 37817281
- PMCID: PMC10566155
- DOI: 10.1186/s13287-023-03521-2
Vascular organoids: unveiling advantages, applications, challenges, and disease modelling strategies
Abstract
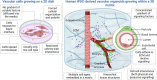
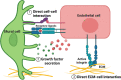
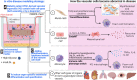
Understanding mechanisms and manifestations of cardiovascular risk factors, including diabetes, on vascular cells such as endothelial cells, pericytes, and vascular smooth muscle cells, remains elusive partly due to the lack of appropriate disease models. Therefore, here we explore different aspects for the development of advanced 3D in vitro disease models that recapitulate human blood vessel complications using patient-derived induced pluripotent stem cells, which retain the epigenetic, transcriptomic, and metabolic memory of their patient-of-origin. In this review, we highlight the superiority of 3D blood vessel organoids over conventional 2D cell culture systems for vascular research. We outline the key benefits of vascular organoids in both health and disease contexts and discuss the current challenges associated with organoid technology, providing potential solutions. Furthermore, we discuss the diverse applications of vascular organoids and emphasize the importance of incorporating all relevant cellular components in a 3D model to accurately recapitulate vascular pathophysiology. As a specific example, we present a comprehensive overview of diabetic vasculopathy, demonstrating how the interplay of different vascular cell types is critical for the successful modelling of complex disease processes in vitro. Finally, we propose a strategy for creating an organ-specific diabetic vasculopathy model, serving as a valuable template for modelling other types of vascular complications in cardiovascular diseases by incorporating disease-specific stressors and organotypic modifications.
Keywords: Angiopathy; Microvascular and macrovascular complications; Organ-specific endothelial dysfunction; Pluripotent stem cells; Vascular disease modelling; Vasculopathy.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Naderi-Meshkin H, Eleftheriadou M, Carney G, Cornelius VA, Nelson C-A, Kelaini S, et al. Impaired function in diabetic patient iPSCs-derived blood vessel organoids stem from a subpopulation of vascular cells. bioRxiv. 2022;2022-07.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

