Current approaches to spinal cord protection during open thoracoabdominal aortic aneurysm repair
- PMID: 37817849
- PMCID: PMC10561332
- DOI: 10.21037/acs-2023-scp-10
Current approaches to spinal cord protection during open thoracoabdominal aortic aneurysm repair
Abstract
Spinal cord deficit (SCD) is a feared complication after thoracoabdominal aortic aneurysm repair. Vigilant management throughout the perioperative period is necessary to reduce the risk of SCD. Measures for preventing SCD during the intraoperative period include preoperative optimization and recognizing patients at a higher risk of SCD. In this manuscript, we discuss intraoperative adjuncts including utilization of cerebrospinal fluid drainage, left heart bypass, mild hypothermia, selective reimplantation of intercostal and lumbar arteries, and renal and visceral vessel perfusion. From the operative to the postoperative period, careful attention to avoiding hypotension and anemia is important. If SCD is recognized early, therapeutic intervention may be implemented to mitigate injury.
Keywords: Spinal cord; aortic aneurysm; perioperative care; thoracoabdominal.
2023 Annals of Cardiothoracic Surgery. All rights reserved.
Conflict of interest statement
Conflicts of Interest: JSC participates in clinical studies with and/or consults for Terumo Aortic, Medtronic, W. L. Gore & Associates, CytoSorbents, Edwards Lifesciences, and Abbott Laboratories and receives royalties and grant support from Terumo Aortic. SAL consults for Terumo Aortic and Cerus and serves as a principal investigator for clinical studies sponsored by Terumo Aortic and CytoSorbents. SAL’s work is supported in part by the Jimmy and Roberta Howell Professorship in Cardiovascular Surgery at Baylor College of Medicine. OP provides consultation for and participates in clinical trials with Medtronic and W.L. Gore & Associates. MRM serves on the advisory board for Medtronic and Edwards Lifesciences. SC has served on advisory boards for Edwards Lifesciences, La Jolla Pharmaceutical Company, Eagle Pharmaceuticals and Baxter Pharmaceuticals. The other authors have no conflicts of interest to declare.
Figures
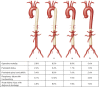
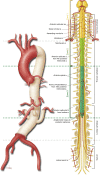
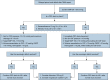

References
Publication types
LinkOut - more resources
Full Text Sources
