Using Ex Situ and In Situ HERFD-XANES to Reveal the Superior Oxidation and Reduction Cycling of Ceria Nanocubes Dispersed in Silica Aerogel
- PMID: 37817919
- PMCID: PMC10561250
- DOI: 10.1021/acs.jpcc.3c03785
Using Ex Situ and In Situ HERFD-XANES to Reveal the Superior Oxidation and Reduction Cycling of Ceria Nanocubes Dispersed in Silica Aerogel
Abstract
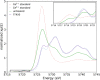
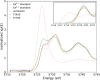
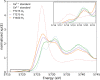
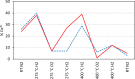
The oxygen storage capacity of ceria-based catalytic materials is influenced by their size, morphology, and surface structure, which can be tuned using surfactant-mediated synthesis. In particular, the cuboidal morphology exposes the most reactive surfaces; however, when the capping agent is removed, the nanocubes can agglomerate and limit the available reactive surface. Here, we study ceria nanocubes, lanthanum-doped ceria nanocubes, and ceria nanocubes embedded inside a highly porous silica aerogel by high-energy resolution fluorescence detection-X-ray absorption near edge spectroscopy at the Ce L3 edge. In situ measurements showed an increased reversibility of redox cycles in ceria nanocubes when embedded in the aerogel, demonstrating enhanced reactivity due to the retention of reactive surfaces. These aerogel nanocomposites show greater improvement in the redox capacity and increased thermal stability of this catalytic material compared to the surfactant-capped nanocubes. Ex situ measurements were also performed to study the effect of lanthanum doping on the cerium oxidation state in the nanocubes, indicating a higher proportion of Ce4+ compared to that of the undoped ceria nanocubes.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Melchionna M.; Fornasiero P. The role of ceria-based nanostructured materials in energy applications. Mater. Today 2014, 17, 349–357. 10.1016/j.mattod.2014.05.005. - DOI
-
- Chang K.; Zhang H.; Cheng M.; Lu Q. Application of Ceria is CO2 Conversion Catalysis. ACS Catal. 2020, 10 (1), 613–631. 10.1021/acscatal.9b03935. - DOI
-
- Mamontov E.; Egami T.; Brezny R.; Koranne M.; Tyagi S. Lattice Defects and Oxygen Storage Capacity of Nanocrystalline Ceria and Ceria-Zirconia. J. Phys. Chem. B 2000, 104, 11110–11116. 10.1021/jp0023011. - DOI
LinkOut - more resources
Full Text Sources
